

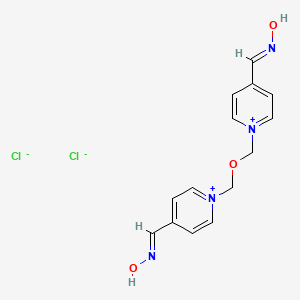

1. Chloride, Obidoxime
2. Obidoxim
3. Obidoxime
4. Toxogonin
1. Obidoxime
2. 114-90-9
3. Obidoxime Dichloride
4. Obidoxim
5. Obidoxime (dichloride)
6. 3hxr312z9m
7. Toksobidin
8. Toxogonine
9. 1,1'-(oxydimethylene)bis(4-formylpyridinium) Dichloride Dioxime
10. Nsc-757237
11. Obidoxime Chloride (usan)
12. Pyridinium, 1,1'-(oxybis(methylene))bis(4-(hydroxyimino)methyl)-, Dichloride
13. Luh(sub 6)
14. Obidoxime Hydrochloride
15. Obidoxime Chloride [usan]
16. Luh6
17. Lueh 6
18. Obidoximi Chloridum
19. Obidoxime Dihydrochloride
20. Toxogonin Dichloride
21. Chlorure D'obidoxime
22. Cloruro De Obidoxima
23. Obidoxime Chloride [usan:inn]
24. Obidoximi Chloridum [inn-latin]
25. Chlorure D'obidoxime [inn-french]
26. Cloruro De Obidoxima [inn-spanish]
27. Einecs 204-059-5
28. Unii-3hxr312z9m
29. Obidoximchlorid
30. Bis(isonicotinaldoxime 1-methyl) Ether Dichloride
31. 1,3-bis(4-aldoximinopyridinium) Dimethyl Ether Bichloride
32. N,n-dimethyleneoxidebis(pyridinium-4-aldoxime) Dichloride
33. Bis(4-hydroxyiminomethylpyridinium-1-methyl)ether Dichloride
34. 1,1'-(oxybis(methylene))bis(4-((hydroxyimino)methyl)pyridin-1-ium) Chloride
35. 1,1'-(oxydimethylene)bis(4-formylpyridinium) Dioxime Dichloride
36. 1,1'-(oxydimethylene)bis(4-formylpyridinium)dichloride Dioxime
37. 1,3-bis(4-hydroxyiminomethyl-1-pyridinio)-2-oxapropane Dichloride
38. N,n-dimethylenoxid-bis-(pyridinium-4-aldoxim)-dichlorid [german]
39. Ether Bis-14-hydroxy-iminomethylopyridine-(1)-metylodichloride [polish]
40. 1,1'-(oxybis(methylene))bis(4-(hydroxyimino)methyl)pyridinium Dichloride
41. Obidoxime Chloride [mi]
42. N,n-dimethylenoxid-bis-(pyridinium-4-aldoxim)-dichlorid
43. Obidoxime Chloride [inn]
44. Obidoxime Chloride [mart.]
45. Ether Bis-14-hydroxy-iminomethylopyridine-(1)-metylodichloride
46. Obidoxime Chloride [who-dd]
47. Mfcd00038864
48. Pyridinium, 1,1'-(oxydimethylene)bis(4-formyl-, Dichloride, Dioxime
49. Akos025397238
50. Akos030241577
51. Cs-w011824
52. Hy-w011108
53. Nsc 757237
54. Obidoxime Chloride, >=95.0% (hplc)
55. D05215
56. Obidoxime Chloride, Analytical Reference Material
57. J-003154
58. 1,1'-(oxybis(methylene))bis(4-((hydroxyimino)methyl)pyridin-1-ium)chloride
59. 1,1'[oxybis(methylene)]-bis[4-(hydroxyimino)methyl]pyridinium Dichloride
60. Pyridinium, 1,1'-[oxybis(methylene)]bis[4-[(hydroxyimino)methyl]-, Chloride (1:2)
| Molecular Weight | 359.2 g/mol |
|---|---|
| Molecular Formula | C14H16Cl2N4O3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 358.0599458 g/mol |
| Monoisotopic Mass | 358.0599458 g/mol |
| Topological Polar Surface Area | 82.2 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 303 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Cholinesterase Reactivators
Drugs used to reverse the inactivation of cholinesterase caused by organophosphates or sulfonates. They are an important component of therapy in agricultural, industrial, and military poisonings by organophosphates and sulfonates. (See all compounds classified as Cholinesterase Reactivators.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB13 - Obidoxime