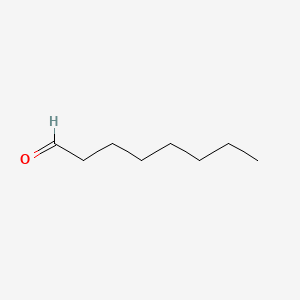

1. Caprylic Aldehyde
1. 124-13-0
2. Caprylaldehyde
3. Caprylic Aldehyde
4. N-octanal
5. 1-octanal
6. N-octyl Aldehyde
7. N-octaldehyde
8. N-caprylaldehyde
9. Octanaldehyde
10. Octyl Aldehyde
11. N-octylal
12. Aldehyde C-8
13. Octanoic Aldehyde
14. Octylaldehyde
15. C-8 Aldehyde
16. Octaldehyde
17. 1-octylaldehyde
18. 1-octaldehyde
19. 1-caprylaldehyde
20. Aldehyde C8
21. N-octanaldehyde
22. Antifoam-lf
23. Oktylaldehyd
24. Oktanal
25. Octanal, Tech.
26. Caprylaldehyd
27. Fema No. 2797
28. Kaprylaldehyd
29. Octylaldehyd
30. Nsc 1508
31. Aldehido C-8
32. Chembl18407
33. Chebi:17935
34. Nsc1508
35. Xge9999h19
36. Nsc-1508
37. Nsc-8969
38. Dsstox_cid_1643
39. Wln: Vh7
40. Dsstox_rid_76257
41. Dsstox_gsid_21643
42. Octanal (natural)
43. Albumin Tannate
44. Octyl Aldehydes
45. Cas-124-13-0
46. Hsdb 5147
47. Einecs 204-683-8
48. Mfcd00007029
49. Brn 1744086
50. N-octylaldehyde
51. Capryl Aldehyde
52. Unii-xge9999h19
53. Ai3-03961
54. N -octanal
55. Octan-1-one
56. Octan-8-one
57. Oya
58. Octanal, 99%
59. Octanal [fcc]
60. N-octanal [fhfi]
61. Bmse000851
62. Ec 204-683-8
63. Octanal, Analytical Standard
64. Octylaldehyde [hsdb]
65. Schembl28601
66. 4-01-00-03337 (beilstein Handbook Reference)
67. Caprylic Aldehyde [mi]
68. Qspl 183
69. Dtxsid3021643
70. Octanal (aldehyde C-8)
71. Nsc8969
72. Octanal, >=95%, Fcc, Fg
73. Hy-n8015
74. Str04459
75. Zinc1529222
76. Tox21_201415
77. Tox21_300337
78. Bdbm50028817
79. Lmfa06000028
80. Akos009031567
81. Octanal, Natural, >=95%, Fcc, Fg
82. Ncgc00247997-01
83. Ncgc00247997-02
84. Ncgc00254427-01
85. Ncgc00258966-01
86. Cs-0138976
87. Ft-0626917
88. Ft-0631629
89. Ft-0631722
90. Ft-0673199
91. O0044
92. En300-19768
93. C01545
94. Q416673
95. J-660019
96. Q-200605
97. 27457-18-7
| Molecular Weight | 128.21 g/mol |
|---|---|
| Molecular Formula | C8H16O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 6 |
| Exact Mass | 128.120115130 g/mol |
| Monoisotopic Mass | 128.120115130 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 59.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07X - Other antidiarrheals
A07XA - Other antidiarrheals
A07XA01 - Albumin tannate
Rats were nose exposed to an atmosphere containing 11.4 ppm of (11)C-octanal for 2 min. Inhaled octanal was absorbed from the lungs in a biphasic manner and the greatest concentration of octanal occurred in most tissues at 5 min. Tissue activities calculated on the basis of the administered dose and on the radiolabel retained until the animal was killed indicated a redistribution of the radiolabel as metabolic products after 20 min. The labeled carbon was eliminated in a biphasic manner as (11)CO2, which accounted for essentially all of the activity lost by the exposed rats.
PMID:6114832 Kutzman RS et al; Drug Metab Dispos 9 (4): 331-3 (1981)