
1. Ach-3102
1. 1415119-52-6
2. Ovr52k7bdw
3. Odalasvir (ach-3102)
4. Ach-3102
5. Ach-0143102
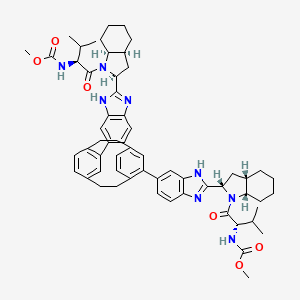
6. Carbamic Acid, N,n'-(tricyclo(8.2.2.24,7)hexadeca-4,6,10,12,13,15-hexaene-5,11-diylbis(1h-benzimidazole-6,2-diyl((2s,3as,7as)-octahydro-1h-indole-2,1-diyl)((1s)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl)))bis-, C,c'-dimethyl Ester
7. Dimethyl N,n'-(1,4(1,4)-dibenzenacyclohexaphane-12,42-diylbis(1hbenzimidazole-5,2-diyl((2s,3as,7as)-octahydro-1h-indole-2,1-diyl)((2s)-3-methyl-1-oxobutan-1,2-diyl)))biscarbamate
8. Dimethyl ((2s,2's)-((2s,2's,3as,3a's,7as,7a's)-(1,4(1,4)-dibenzenacyclohexaphane-12,43-diylbis(1h-benzo[d]imidazole-6,2-diyl))bis(octahydro-1h-indole-2,1-diyl))bis(3-methyl-1-oxobutane-1,2-diyl))dicarbamate
9. Methyl N-[(2s)-1-[(2s,3as,7as)-2-[6-[11-[2-[(2s,3as,7as)-1-[(2s)-2-(methoxycarbonylamino)-3-methylbutanoyl]-2,3,3a,4,5,6,7,7a-octahydroindol-2-yl]-3h-benzimidazol-5-yl]-5-tricyclo[8.2.2.24,7]hexadeca-1(12),4,6,10,13,15-hexaenyl]-1h-benzimidazol-2-yl]-2,3,3a,4,5,6,7,7a-octahydroindol-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate
10. Odalasvir [usan:inn]
11. Unii-ovr52k7bdw
12. Odalasvir [inn]
13. Odalasvir [usan]
14. Odalasvir [who-dd]
15. Chembl3544977
16. Schembl14122808
17. Schembl18685009
18. Schembl19236067
19. Dtxsid401032258
20. Db13041
21. Q4650666
| Molecular Weight | 1001.3 g/mol |
|---|---|
| Molecular Formula | C60H72N8O6 |
| XLogP3 | 11.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Exact Mass | 1000.55748205 g/mol |
| Monoisotopic Mass | 1000.55748205 g/mol |
| Topological Polar Surface Area | 175 Ų |
| Heavy Atom Count | 74 |
| Formal Charge | 0 |
| Complexity | 1840 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
