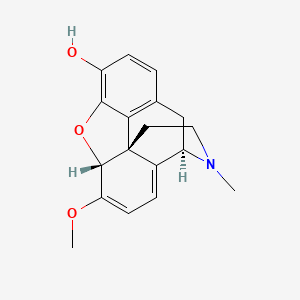

1. 6-demethoxythebaine
2. O(3)-dimethylthebaine
3. Oripavine Hydrochloride, (5alpha)-isomer
1. 467-04-9
2. 575aou51cr
3. Chebi:7782
4. Einecs 207-385-6
5. Brn 0046094
6. Unii-575aou51cr
7. 6,7,8,14-tetradehydro-4,5alpha-epoxy-6-methoxy-17-methylmorphinan-3-ol (oripavine)
8. 3-o-demethylthebaine
9. Oripavine [mi]
10. (5alpha)-6,7,8,14-tetradehydro-4,5-epoxy-6-methoxy-17-methylmorphinan-3-ol
11. 6,7,8,14-tetradehydro-4,5-alpha-epoxy-6-methoxy-17-methyl-morphinan-3-ol
12. Schembl37889
13. 4-27-00-02270 (beilstein Handbook Reference)
14. Chembl437602
15. Ids-no-010
16. Dea No. 9330
17. Schembl19880919
18. Hsdb 8324
19. Dtxsid10196908
20. Morphinan-3-ol, 6,7,8,14-tetradehydro-4,5-epoxy-6-methoxy-17-methyl-, (5.alpha.)-
21. Oripavine 0.1 Mg/ml In Methanol
22. (4r,7ar,12bs)-7-methoxy-3-methyl-2,4,7a,13-tetrahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-9-ol
23. Morphinan-3-ol, 6,7,8,14-tetradehydro-4,5-alpha-epoxy-6-methoxy-17-methyl-
24. C06175
25. Morphine Sulfate Impurity C [ep Impurity]
26. Q420639
27. Codeine Monohydrate Impurity L [ep Impurity]
28. Codeine Hydrochloride Dihydrate Impurity L [ep Impurity]
29. Codeine Phosphate Hemihydrate Impurity L [ep Impurity]
30. 6,7,8,14-tetradehydro-4,5.alpha.-epoxy-6-methoxy-17-methylmorphinan-3-ol
31. (1s,5r,13r)-14-methoxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0?,??.0?,??.0?,??]octadeca-7,9,11(18),14,16-pentaen-10-ol
| Molecular Weight | 297.3 g/mol |
|---|---|
| Molecular Formula | C18H19NO3 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 297.13649347 g/mol |
| Monoisotopic Mass | 297.13649347 g/mol |
| Topological Polar Surface Area | 41.9 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 571 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
1. Codeine O-demethylation to morphine is mediated by cytochrome P450 IID1 (rat), or P450 IID6 (man), and exhibits genetic polymorphism. Thebaine is a precursor in the formation of endogenous morphine and codeine in man, being O-demethylated to oripavine. 2. The objective of the present study was to ascertain whether the O-demethylation of thebaine to oripavine was mediated by cytochrome P450 IID1 in rat liver microsomes. 3. Thebaine O-demethylation showed strain differences in female Sprague-Dawley (SD) and female Dark-Agouti (DA) rats, which serve as a model for the human debrisoquine/sparteine metabolism phenotypes. 4. The total intrinsic clearance of thebaine to oripavine was high (19.7 ml/h per mg protein) in SD rats, indicating that oripavine is a major metabolite of thebaine. A 3-fold lower intrinsic clearance was observed in DA rats (6.7 ml/h per mg protein). 5. Thebaine O-demethylation was inhibited by quinine and known substrates of cytochrome P450 IID1/P450 IID6, supporting the major involvement of cytochrome P450 IID1 in oripavine formation in rats.
PMID:1763524 Mikus G et al; Xenobiotica 21 (11): 1501-9 (1991)
Thebaine, an intermediate of morphine biosynthesis in the poppy plant, Papaver somniferum, was transformed to oripavine, codeine, and morphine by rat liver, kidney, and brain microsomes in the presence of an NADPH-generating system. The formation of morphine, codeine, and oripavine was identified by a specific RIA, HPLC, and GCMS. Thebaine also gave rise to four other compounds, which for the moment are unidentified. NADH dramatically increased the formation of both codeine and morphine when used together with an NADPH-generating system, especially in liver microsomes. NADPH is essential in the formation of oripavine from thebaine and morphine from codeine, while NADH is critical in the conversion of thebaine to codeine and from oripavine to morphine. Carbon monoxide or SKF 525A inhibited the conversion, indicating a role of cytochrome P-450. These results provide evidence for the enzymatic in vitro conversion by mammalian tissues of thebaine to morphine. The pathway is similar to that which exists in plants.
PMID:3422490 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC279748 Kodaira H, Spector S; Proc Natl Acad Sci U S A 85 (4): 1267-71 (1988)