


1. Citrate, Norflex Orphenadrine
2. Citrate, Orphenadrine
3. Disipal
4. Hydrochloride, Orphenadrine
5. Lysantin
6. Mefenamine
7. Mefenamine, Sodium
8. Mephenamine
9. Methyldiphenylhydramine
10. Norflex
11. Norflex Orphenadrine Citrate
12. Orphenadrine
13. Orphenadrine Citrate
14. Orphenadrine Citrate, Norflex
15. Sodium Mefenamine
1. 341-69-5
2. Orphenadrine Hcl
3. Mephenamin
4. Disipal
5. Mebedrol
6. Mephenamine
7. Orphenadrine (hydrochloride)
8. Brocadisipal
9. Mephenamin Hydrochloride
10. Orfenadrin Hydrochloride
11. Mephenamine Hydrochloride
12. Mefenamin Hydrochloride
13. Bf 5930
14. Bg 5930
15. Bs 5930
16. Orphenadrine (chloride)
17. Nsc-82357
18. Uby910duxh
19. Mls000069427
20. N,n-dimethyl-2-(2-methylbenzhydryloxy)ethylamine Hydrochloride
21. 2-dimethylaminoethyl 2-methylbenzhydryl Ether Hydrochloride
22. Beta-dimethylaminoethyl 2-methylbenzhydryl Ether Hydrochloride
23. Smr000058999
24. Mephenamin Forte
25. Ethanamine, N,n-dimethyl-2-((2-methylphenyl)phenylmethoxy)-, Hydrochloride
26. Ethanamine, N,n-dimethyl-2-[(2-methylphenyl)phenylmethoxy]-, Hydrochloride
27. Chebi:60902
28. Sr-01000002975
29. Einecs 206-435-4
30. Unii-uby910duxh
31. Nsc 82357
32. Brocasipal
33. N,n-dimethyl-2-[(2-methylphenyl)-phenylmethoxy]ethanamine;hydrochloride
34. Prestwick_663
35. Disipal (tn)
36. Disipal Hydrochloride
37. Mfcd00012480
38. Orphenadrine Chloride
39. Opera_id_658
40. Orphenedrine Hydrochloride
41. Dsstox_cid_5815
42. Cas-341-69-5
43. Dsstox_rid_77934
44. Dsstox_gsid_25815
45. Regid_for_cid_9568
46. Mls001148572
47. Mls002548882
48. Mls006011645
49. Schembl1235378
50. Chembl1201023
51. Dtxsid5025815
52. Orphenadrine-[d3] Hydrochloride
53. Hms1568p19
54. Hy-b1126
55. Nsc82357
56. Tox21_301927
57. Tox21_500884
58. S5664
59. Akos024307520
60. Wln: 1n1&2oyr&r B1 &gh
61. Ccg-220239
62. Ccg-222188
63. Cs-4723
64. Lp00884
65. Nc00465
66. Ethylamine, N,n-dimethyl-2-((o-methyl-alpha-phenylbenzyl)oxy)-, Hydrochloride
67. Ncgc00089814-04
68. Ncgc00094204-01
69. Ncgc00094204-02
70. Ncgc00255176-01
71. Ncgc00261569-01
72. Ls-14663
73. Orphenadrine Hydrochloride [mart.]
74. Orphenadrine Hydrochloride [vandf]
75. Orphenadrine Hydrochloride [who-dd]
76. Db-048578
77. Eu-0100884
78. Ft-0631984
79. O0406
80. Orphenadrine Hydrochloride, >=98.0% (at)
81. Wln: 1n1 & 2oyr & R B1 & Gh
82. D02599
83. O 3752
84. Orphenadrine Hydrochloride [ep Impurity]
85. Orphenadrine Hydrochloride [orange Book]
86. Orphenadrine Hydrochloride [ep Monograph]
87. J-019475
88. Sr-01000002975-2
89. Sr-01000002975-7
90. Q27129640
91. N,n-dimethyl-2-(phenyl(o-tolyl)methoxy)ethanamine Hydrochloride
92. Ethylamine,n-dimethyl-2-[phenyl-(o-tolyl)methoxy]-, Hydrochloride
93. N,n-dimethyl-2-[(.alpha.-o-tolylbenzyl)oxy]ethylamine Hydrochloride
94. N,n-dimethyl-2-[.alpha.-(o-tolyl)benzyloxy]ethylamine Hydrochloride
95. Ethanamine,n-dimethyl-2-[(2-methylphenyl)phenylmethoxy]-, Hydrochloride
96. Ethylamine,n-dimethyl-2-[.alpha.-(o-tolyl)benzyloxy]-, Hydrochloride
97. N,n-dimethyl-2-(o-methyl-.alpha.-phenylbenzyloxy)ethylamine Hydrochloride
98. Orphenadrine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
99. Ethylamine,n-dimethyl-2-[(o-methyl-.alpha.-phenylbenzyl)oxy]-, Hydrochloride
100. Orphenadrine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
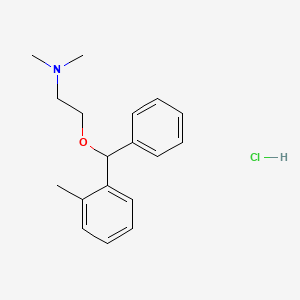
| Molecular Weight | 305.8 g/mol |
|---|---|
| Molecular Formula | C18H24ClNO |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 305.1546421 g/mol |
| Monoisotopic Mass | 305.1546421 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 260 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Cytochrome P-450 CYP2B6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2B6. (See all compounds classified as Cytochrome P-450 CYP2B6 Inhibitors.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
N - Nervous system
N04 - Anti-parkinson drugs
N04A - Anticholinergic agents
N04AB - Ethers chemically close to antihistamines
N04AB02 - Orphenadrine (chloride)
