


1. Oteracil
1. Oteracil
2. 937-13-3
3. 5-azaorotic Acid
4. Allantoxanic Acid
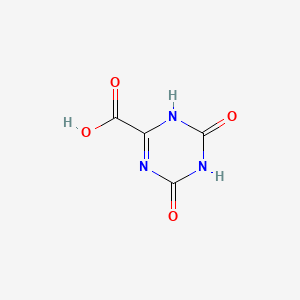
5. 4,6-dioxo-1,4,5,6-tetrahydro-1,3,5-triazine-2-carboxylic Acid
6. Oteracil [inn]
7. Oxonate
8. 1,4,5,6-tetrahydro-4,6-dioxo-1,3,5-triazine-2-carboxylic Acid
9. 4,6-dioxo-1h-1,3,5-triazine-2-carboxylic Acid
10. 5vt6420tig
11. Chebi:30863
12. 1,4,5,6-tetrahydro-4,6-dioxo-s-triazine-2-carboxylic Acid
13. Oxc
14. Unii-5vt6420tig
15. Oxonsaure
16. Oxonic-acid
17. 1,3,5-triazine-2-carboxylic Acid, 1,4,5,6-tetrahydro-4,6-dioxo-
18. Oxonic Acid [mi]
19. Oteracil [who-dd]
20. Nciopen2_000442
21. Oteracil [ema Epar]
22. Schembl464773
23. Chembl181932
24. Dtxsid9048358
25. Bcp32168
26. Zinc13514753
27. Akos006272679
28. Am84784
29. Db03209
30. Sb73387
31. Db-079670
32. Dihydroxy-1,3,5-triazine-2-carboxylic Acid
33. Ft-0707227
34. S-triazine-2,4-dione-6-carboxylic Acid
35. Q22075725
36. 1,3,5-triazine-2-carboxylicacid,1,4,5,6-tetrahydro-4,6-dioxo-
37. 4,6-dioxo-1,4,5,6-tetrahydro-1,3,5-triazine-2-carboxylicacid
38. Oteracil Pound>>5-azaorotic Acid Pound>>4,6-dioxo-1,4,5,6-tetrahydro-1,3,5-triazine-2-carboxylic Acid
| Molecular Weight | 157.08 g/mol |
|---|---|
| Molecular Formula | C4H3N3O4 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 157.01235559 g/mol |
| Monoisotopic Mass | 157.01235559 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 269 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oteracil is used as an adjunct to antineoplastic therapy. When used within the product Teysuno, oteracil is indicated for the treatment of adults with advanced gastric (stomach) cancer when given in combination with cisplatin.
Absorption
After administration of a single dose of 50 mg Teysuno (expressed as tegafur content), median Tmax for Teysuno components tegafur, gimeracil, and oteracil was 0.5, 1.0, and 2.0 hours, respectively.
Route of Elimination
Following a single dose of Teysuno, approximately 3.8% to 4.2% of administered tegafur, 65% to 72% of administered gimeracil, and 3.5% to 3.9% of administered oteracil were excreted unchanged in the urine.
Volume of Distribution
Although no intravenous data are available for Teysuno in humans, the volume of distribution could be roughly estimated from the apparent volume of distribution and urinary excretion data as 16 l/m2, 17 l/m2, and 23 l/m2 for tegafur, gimeracil and oteracil, respectively.
Based on the results of in vitro studies, a part of oteracil is non-enzymatically degraded to 5-azauracil (5-AZU) by gastric fluid, and is then converted to cyanuric acid (CA) in the digestive tract. Only a small amount of oteracil is metabolised in the liver because of its low permeability.
Following a single dose of Teysuno, T1/2 values ranged from 6.7 to 11.3 hours for tegafur, from 3.1 to 4.1 hours for gimeracil, and from 1.8 to 9.5 hours for oteracil.
Oteracil's main role within Teysuno is to reduce the activity of 5-FU within normal gastrointestinal mucosa, and therefore reduce's gastrointestinal toxicity. It functions by blocking the enzyme orotate phosphoribosyltransferase (OPRT), which is involved in the production of 5-FU.