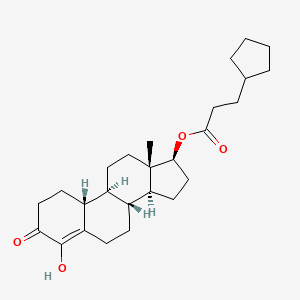

1. 4,17beta-dihydroxy-estr-4-en-3-one 17-cyclopentylpropionate
2. Oxabolone Cypionate
1. Oxabolone Cypionate
2. 1254-35-9
3. Oxabolone Cipioncate
4. Fi 5852
5. Oxabolone Cipionate [inn]
6. 5rxy50q01n
7. Oxabolone 17-cyclopentanepropionate
8. Nsc-522767
9. Fi-5852
10. Oxaboloni Cipionas
11. Esterbol Depo
12. Oxaboloni Cypionas
13. Ossabolone Cipionato
14. Cipionate D'oxabolone
15. Cipionato De Oxabolona
16. Unii-5rxy50q01n
17. Ossabolone Cipionato [dcit]
18. Oxaboloni Cipionas [inn-latin]
19. Ncgc00181156-01
20. Cipionate D'oxabolone [inn-french]
21. Einecs 215-011-8
22. Cipionato De Oxabolona [inn-spanish]
23. Dsstox_cid_26879
24. Dsstox_rid_81983
25. Dsstox_gsid_46879
26. [(8r,9s,10r,13s,14s,17s)-4-hydroxy-13-methyl-3-oxo-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl] 3-cyclopentylpropanoate
27. Schembl4821339
28. Oxabolone Cipionate (jan/inn)
29. Chembl2105318
30. Dtxsid3046879
31. Chebi:31940
32. Oxabolone Cipionate [jan]
33. 4,17beta-dihydroxy-estr-4-en-3-one 17-cyclopentylpropionate
34. Oxabolone Cipionate [mart.]
35. Tox21_112760
36. Oxabolone Cipionate [who-dd]
37. Zinc26892649
38. Db13185
39. Nsc 522767
40. Cas-1254-35-9
41. 4-hydroxy-19-nortestosterone 17beta-cypionate
42. D01149
43. Oxabolone 17-cyclopentanepropionate [mi]
44. Q7115043
45. 4,17beta-dihydroxyestr-4-en-3-one 17-cyclopentanepropionate
46. 4,17.beta.-dihydroxyestr-4-en-3-one 17-cyclopentanepropionate
47. (8r,9s,13s,14s,17s)-4-hydroxy-13-methyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl 3-cyclopentylpropanoate
| Molecular Weight | 414.6 g/mol |
|---|---|
| Molecular Formula | C26H38O4 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 414.27700969 g/mol |
| Monoisotopic Mass | 414.27700969 g/mol |
| Topological Polar Surface Area | 63.6 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 733 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used as a performance enhancing drug illicitly in athletes.
Androgenic effects include enhanced secondary sexual characteristics.
A - Alimentary tract and metabolism
A14 - Anabolic agents for systemic use
A14A - Anabolic steroids
A14AB - Estren derivatives
A14AB03 - Oxabolone cipionate
Route of Elimination
The metabolites and unchanged form of oxabolone can be detected in the urine.
Clearance
The elimination on the first day following intravenous injection of oxabolone cipionate is slow and reaches a rapid excretion phase with the maximum urinary level in the fifth day of administration.
The prodrug oxabolone cipionate is converted to oxabolone. Oxabolone is then metabolized into 4-Hydroxyestr-4-en-3,17-dione (M2) which is the most abundant metabolite via oxidation of the 17-hydroxyl group. 4-Hydroxyestran-3,17-dione (M1) is formed by reduction of the A ring double bond along with the oxidation of the 17-hydroxyl group. Three isomeric compounds exist as another metabolite, as the 3,4-dihydroxy-5-estran-17-one, 3,4-dihydroxy-5-estran-17-one, and 3,4-dihydroxy-5-estran-17-one.