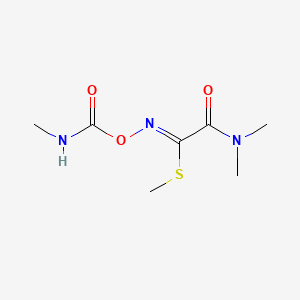

1. Dpx 1410
2. N',n'-dimethyl-n-((methylcarbamoyl)oxy)-1- Thiooxamimidate
3. Oxamyl 1211
4. Oxamyl 4509
5. Vydate
1. 23135-22-0
2. Dioxamyl
3. N,n-dimethyl-alpha-methylcarbamoyloxyimino-alpha-(methylthio)acetamide
4. Ethanimidothioic Acid, 2-(dimethylamino)-n-[[(methylamino)carbonyl]oxy]-2-oxo-, Methyl Ester
5. 2-dimethylamino-1-(methylthio)glyoxal O-methylcarbamoylmonoxime
6. Vydate L
7. Methyl (1z)-2-(dimethylamino)-n-(methylcarbamoyloxy)-2-oxoethanimidothioate
8. 2-(dimethylamino)-n-(((methylamino)carbonyl)oxy)-2-oxoethanimidothioic Acid Methyl Ester
9. N',n'-dimethylcarbamoyl(methylthio)methylenamine N-methylcarbamate
10. Du Pont 1410
11. S-methyl 1-(dimethylcarbamoyl)-n-((methylcarbamoyl)oxy)thioformimidate
12. Oxamyl, Analytical Standard
13. Insecticide-nematicide 1410
14. Vydate L Insecticide/nematicide
15. Dpx 1410l
16. Methyl 2-(dimethylamino)-n-(methylcarbamoyloxy)-2-oxoethanimidothioate
17. N',n'-dimethyl-n-((methylcarbamoyl)oxy)-1-methylthio-oxamimidic Acid
18. S-methyl N',n'-dimethyl-n-(methylcarbamoyloxy)-1-thio-oxamimidate
19. Vydate L Oxamyl Insecticide/nematocide
20. Nsc 379588
21. Chebi:38539
22. Methyl N',n'-dimethyl-n-((methylcarbamoyl)oxy)-1-thiooxamimidate
23. S-methyl 1-(dimethylcarbamoyl)-n-[(methylcarbamoyl)oxy]thioformimidate
24. Nsc-379588
25. Methyl (1z)-2-(dimethylamino)-n-{[(methylamino)carbonyl]oxy}-2-oxoethanimidothioate
26. Oxamimidic Acid, N',n'-dimethyl-n-[(methylcarbamoyl)oxy]-1-thio-, Methyl Ester
27. Oxamyl (pesticide)
28. Nematicide 1410
29. Schembl1765917
30. Chembl2140710
31. N,n-dimethyl-.alpha.-methylcarbamoyloxyimino-.alpha.-(methylthio)acetamide
32. Oxamimidic Acid, N',n'-dimethyl-n-((methylcarbamoyl)oxy)-1-methylthio-
33. Dtxsid10860293
34. Yaa13522
35. Zinc1590888
36. Nsc379588
37. Akos015898748
38. D 1410
39. Ncgc00163853-01
40. Ncgc00163853-02
41. Ncgc00163853-03
42. Ncgc00163853-04
43. Pesticide2_oxamyl_c7h13n3o3s_vydate
44. Oxamyl, Pestanal(r), Analytical Standard
45. C18419
46. Oxamyl 10 Microg/ml In Methyl-tert-butyl Ether
47. 135o220
48. W-110557
49. Methyl (1z)-2-(dimethylamino)-n-[(methylcarbamoyl)oxy]-2-oxoethanimidothioate
50. Methyl 2-(dimethylamino)-n-([(methylamino)carbonyl]oxy)-2-oxoethanimidothioate #
51. Oxamylmethyl (1z)-2-(dimethylamino)-n-{[(methylamino)carbonyl]oxy}-2-oxoethanimidothioate
52. 32817-80-4
| Molecular Weight | 219.26 g/mol |
|---|---|
| Molecular Formula | C7H13N3O3S |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 219.06776246 g/mol |
| Monoisotopic Mass | 219.06776246 g/mol |
| Topological Polar Surface Area | 96.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
When (14)C-oxamyl was administered to lactating goats, most of the dose was rapidly eliminated via urine and feces. Some (14)CO2 also formed. No intact oxamyl or related metabolites were detected in milk, blood, urine or tissues; however, (14)C was incorporated into lactose, casein, triglyceride fats, and amino acids of protein in blood and tissues.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 409
Following ip injection of (14)C oxamyl, mice excreted 75.5% of the activity within 6 hours. By 96 hours, 88.7% had been excreted in the urine and 7.7% in the feces. At 6 and 72 hours after injection, organosoluble radioactive material constituted 24.7 and 6.5%, respectively, of the total radioactivity in the urine. The organosoluble cmpds identified included oxamyl and methyl N'-methyl-N-(methyl(carbomyl)oxy)-1-thiooxamimidate ...
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1168
Little information is available on the distribution of carbamates in the various organs and tissues in mammals following exposure by inhalation or the oral route. The organs in which residues have been reported are the liver, kidneys, brain, fat, and muscle. The half-life in the rat is of the order of 3-8 hr. It seems that the excretion of carbamates via urine is also rapid in man, and that the metabolic pathways in man are the same as those in the rat. /Carbamate pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of July 3,2003: https://www.inchem.org/pages/ehc.html
In a rat metabolism study, SD rats (5 animals/sex/group) received a single oral dose of 14C-oxamyl (1 mg/kg) by gavage. Approximately 80% of the administered radioactivity was eliminated in the urine after 24 hours of dosing, and approximately 91% of the dose was eliminated in the urine by 168 hours. Less than 3% of the dose was found in the feces, and approximately 1% of the dose was found in the carcass. Except for muscle and skin, less than 1% of the dose was found in any tissue examined. The data indicated that oxamyl was readily absorbed with oral administration and rapidly metabolized and eliminated in the urine. There was no sex difference m the pattern of elimination, and there was essentially no sequestration of oxamyl or its metabolites in any tissue examined.
USEPA/Office of Pesticide Programs; Amended Toxicology Chapter for the Registration Eligibility Decision Document - Oxamyl Available from, as of June 18, 2003: https://www.epa.gov/oppsrrd1/reregistration/oxamyl/
The metabolic fate of (1-14C)oxamyl in a lactating goat was investigated. The test animal was administered five consecutive daily doses orally at 31 ppm oxamyl dietary burden. Most of the radioactivity was eliminated via urine (45.3%) and feces (7.2%). (14C)Oxamyl equivalents in edible tissues (liver, kidney, muscle, and fat) and in milk accounted for 6.7 and 10.2% of the dose, respectively. A small percentage (1.9%) of the dose was exhaled as volatile metabolites (primarily 14CO2). No oxamyl nor any closely related metabolites were detected in tissues, milk, or urine. Extensive degradation/metabolism of (1-14C)oxamyl was observed. Radioactive thiocyanate was the major metabolite identified in the milk as well as in the methanol/water extracts for all tissue samples.
Li Y et al; Journal of Agric and Food Chem 45 (3): 962-966 (1997)
The metabolism of oxamyl ... has not been much studied in humans. Oxime production would be expected to occur again ... with subsequent ketone formation as for methomyl. The dimethylacetyl group may hydrolyze to form N,N-dimethylformamide, excreted as N-methylformamide and formamide in urine ... or N,N-dimethyl carbamic acid.
Que Hee, S. (ed.). Biological Monitoring an Introduction. New York, NY: Van Nostrand Reinhold Co., 1993., p. 495
All three of the oxime carbamates, aldicarb, methomyl, and oxamyl, will produce the N-methylformamide ... and N-methylamino formic acid ... as a result of R1 cleavage. The former is a urinary biological monitoring marker for N,N-dimethylformamide ... .
Que Hee, S. (ed.). Biological Monitoring an Introduction. New York, NY: Van Nostrand Reinhold Co., 1993., p. 496
(14)C-oxamyl was degraded by two major pathways in rats that received 1 mg each (2.5 to 4.6 mg/kg) after having received nonradioactive oxamyl for 18 days or more at a dietary level of 50 to 150 ppm (about 2.5 to 7.4 mg/kg/day). Oxamyl was hydrolyzed to an oximino metabolite (methyl N-hydroxy-N',N'-dimethyl-1-thioxamimidate) or converted enzymatically via N,N-dimethyl-1-cyanoformide (DMCF) to N,N-dimethyloxamic acid. Conjugates of the oximino cmpd, the acid, and their monomethyl derivatives constituted over 70% of the metabolites excreted in the urine and feces. Less than 0.3% of the oxamyl was exhaled as carbon dioxide, but incorporation of (14)C-CO2 accounted for more than 50% of the radioactivity remaining in the tissues ... .
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1169
The major component present in the urine was glucuronide of oxime (31 - 37% of the dose), metabolite oxime (13 - 18% of the dose) and the parent oxamyl (7 - 11% of the dose).
USEPA/Office of Pesticide Programs; Amended Toxicology Chapter for the Registration Eligibility Decision Document - Oxamyl Available from, as of June 18, 2003: https://www.epa.gov/oppsrrd1/reregistration/oxamyl/
For more Metabolism/Metabolites (Complete) data for OXAMYL (9 total), please visit the HSDB record page.
Carbamates are effective insecticides by virtue of their ability to inhibit acetylcholinesterase (AChE) in the nervous system. They also inhibit other esterases. The carbamylation of the enzyme is unstable, and the regeneration of AChE is relatively rapid compared with that from a phosphorylated enzyme. Thus, carbamate pesticides are less dangerous with regard to human exposure than organophosphorus pesticides. The ratio between the dose required to produce death and the dose required to produce minimum symptoms of poisoning is substantially larger for carbamate compounds than for organophosphorus compounds. /Carbamate Pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of July 3,2003: https://www.inchem.org/pages/ehc.html
The carbamates alone weakly activated estrogen- or progesterone-responsive reporter genes in breast and endometrial cancer cells. All of the carbamates decreased estradiol- or progesterone-induced reporter gene activity in the breast and endometrial cancer cells. In whole cell competition binding assays, the carbamates demonstrated a limited capacity to displace radiolabeled estrogen or progesterone from /estrogen receptor/ or /progesterone receptor/. /Carbamates/
PMID:9126867 Klotz D et al; Life Sciences 60 (17): 1467-1475 (1997)