





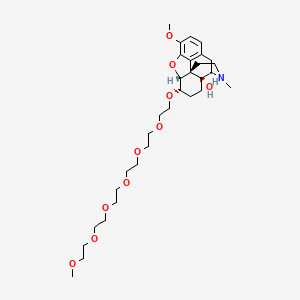

1. 4,5-epoxy-6-(3,6,9,12,15,18-hexaoxanonadec-1-yloxy)-3-methoxy-17-methylmorphinan-14-ol
2. Nktr-181
1. Oxycodegol
2. Oxycodegol [usan]
3. Nktr-181
4. J2wiv0jmml
5. Oxycodegol (usan)
6. 1211231-76-3
7. Oxicodegol
8. Loxicodegol [inn]
9. Unii-j2wiv0jmml
10. Oxycodegol [inn]
11. Loxicodegol (deleted Inn)
12. Loxicodegol [who-dd]
13. Nktr181
14. Gtpl10652
15. Who 9971
16. Db14146
17. Cs-0033429
18. D11424
19. (4r,4as,7s,7ar,12bs)-9-methoxy-7-[2-[2-[2-[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]-3-methyl-1,2,4,5,6,7,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-4a-ol
20. 4,5.alpha.-epoxy-6.alpha.-((2,5,8,11,14,17-hexaoxanonadecan-19-yl)oxy)-3-methoxy-17-methylmorphinan-14-ol
21. 4,5alpha-epoxy-6alpha-((2,5,8,11,14,17-hexaoxanonadecan-19-yl)oxy)-3-methoxy-17-methylmorphinan-14-ol
22. Morphinan-14-ol, 4,5-epoxy-6-(3,6,9,12,15,18-hexaoxanonadec-1-yloxy)-3-methoxy-17-methyl-, (5.alpha.,6.alpha.)-
| Molecular Weight | 595.7 g/mol |
|---|---|
| Molecular Formula | C31H49NO10 |
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 20 |
| Exact Mass | 595.33564676 g/mol |
| Monoisotopic Mass | 595.33564676 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 808 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Loxicodegol was developed as an opioid analgesic with low abuse potential for use in treating chronic pain. An application has been filed for FDA approval of Loxicodegol for use in treating chronic lower back pain.
Loxicodegol displays full analgesic activity comparable to that of oxycodone, producing similar acetone-writhing test responses in mice and hot-plate test maximal latencies in rats. The safety profile of Loxicodegol is much improved from that of other opioid analgesics. The incidence of respiratory depression and sedation is reduced compared to oxycodone and morphine. Generalized pruritus occurred in 7.3% and 4.9% of subjects at 200mg and 400mg of Loxicodegol compared to 41.5% with 40mg oxycodone. Nausea occurred in 2.5%, 2.4%, and 7.3% with 100mg, 200mg, and 400mg of Loxecodegol compared to 29.3% with 40mg oxycodone. Lastly, vomiting occurred in 2.4% with both 200mg and 400mg Loxicodegol compared to 24.4% with 40mg oxycodone. Loxicodegol is much less addictive than other opioid analgesics. It produces a only a slight high at dosages of 400mg, peaking at scores only 25% that of oxycodone, with no difference compared to placebo at lower dosages. No differences have been noted between Loxicodegol and saline in self-administration or behavior reinforcement in monkeys or rats
Absorption
Loxicodegol has a Tmax of 1.8h and a bioavailability of 34% with oral administration. It enters the brain about 17-70 times more slowly than oxycodone. Loxicodegol is also a p-glycoprotein substrate further reducing its transport into the brain.
Volume of Distribution
Loxicodegol has a steady-state Vd of 4.19 L/kg.
Clearance
Loxicodegol has a clearance rate of 59.3 mL/min/kg.
Loxicodegol has a half-life of 4.53 h allowing for a longer duration of action.
Loxicodegol is a full agonist at the -opioid receptor. It also displays selectivity towards this subtype with an affinity of 237 nM compared to 4150 nM and >100000 nM for the - and -opioid receptors. The -opioid receptor is activated with an EC of 12.5 M. Activation of this receptor produces an inhibitory effect on neurotransmission through Gi/Go coupling. Opioid medications like Loxicodegol inhibit voltage gated Ca channel opening presynaptically on C fibres and activate inward-rectifying K channels post-synaptically on 2nd order neurons, leading to hyperpolarization. Together, these prevent the nociceptive signal from traveling up the spinal cord to the brain. In the brain, opioids work through a mechanism of inhibition of inhibition to allow regulatory neurons to suppress nociception.
