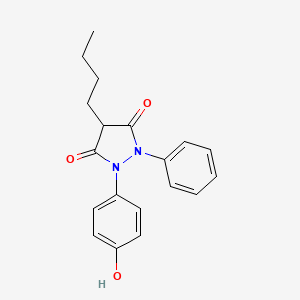

1. Diflamil
2. Hydroxyphenylbutazone
3. Oxyphenylbutazone
4. Tanderil
1. 129-20-4
2. Oxyphenylbutazone
3. Tandearil
4. Oxazolidin
5. Tanderil
6. Oxifenylbutazon
7. Oxiphenbutazone
8. Butapirone
9. Crovaril
10. Flamaril
11. Flogitolo
12. Flogoril
13. Rapostan
14. Visubutina
15. Frabel
16. Oxalid
17. Oxyphenobutazone
18. P-oxyphenylbutazone
19. Oxi-fenibutol
20. Neo-farmadol
21. Artroflog
22. Butaflogin
23. Butanova
24. Butazonic
25. Butilene
26. Californit
27. Deflogin
28. Etrozolidina
29. Flanaril
30. Floghene
31. Flogistin
32. Flogodin
33. Flogostop
34. Flopirina
35. Idrobutazina
36. Infammil
37. Ipabutona
38. Isobutazina
39. Isobutil
40. Offitril
41. Oxibutol
42. Oxybuton
43. Oxyphentamin
44. Pirabutina
45. Piraflogin
46. Poliflogil
47. Tandacote
48. Tandalgesic
49. Tendearil
50. Flogal
51. Infamil
52. Iridil
53. Mysite
54. Neofen
55. Optimal
56. Remazin
57. Reumox
58. Rumapax
59. Telidal
60. Valioil
61. Metabolite I
62. Oxazolidin-geigy
63. P-hydroxyphenylbutazone
64. Oxiphenbutazonum
65. Usaf Ge-14
66. Hydroxyphenylbutazon
67. Oxifenbutazona
68. Tanderal
69. Oxyphenbutazonum
70. Oxyphenbutazone [inn]
71. 3,5-pyrazolidinedione, 4-butyl-1-(4-hydroxyphenyl)-2-phenyl-
72. Oxyphenbutazone Anhydrous
73. Bm 1
74. 1-phenyl-2-(p-hydroxyphenyl)-3,5-dioxo-4-butylpyrazolidine
75. 4-butyl-1-(4-hydroxyphenyl)-2-phenyl-3,5-pyrazolidinedione
76. 1-(p-hydroxyphenyl)-2-phenyl-4-butyl-3,5-pyrazolidinedione
77. 4-butyl-1-(p-hydroxyphenyl)-2-phenyl-3,5-pyrazolidinedione
78. Suganril
79. 4-butyl-1-(4-hydroxyphenyl)-2-phenylpyrazolidine-3,5-dione
80. 4-butyl-2-(4-hydroxyphenyl)-1-phenyl-3,5-dioxopyrazolidine
81. 4-butyl-2-(p-hydroxyphenyl)-1-phenyl-3,5-pyrazolidinedione
82. 1-phenyl-2-(p-hydroxyphenyl)-3,5-dioxo-4-n-butylpyrazolidine
83. 3,5-dioxo-1-phenyl-2-(p-hydroxyphenyl)-4-n-butylpyrazolidene
84. G 27202
85. Oxyphenyl Butazone
86. 3,5-pyrazolidinedione, 4-butyl-1-(p-hydroxyphenyl)-2-phenyl-
87. Mls003373849
88. Flegmostam
89. Oxazolioin
90. Reunabutal
91. Aradinum
92. Butanora
93. Portoril
94. Telidac
95. Chebi:76258
96. Genal
97. A7d84513gv
98. Cinophen-n
99. Nsc-526053
100. Oxyphenbutazone (inn)
101. 1-(p-hydroxyphenyl)-2-phenyl-3,5-dioxo-4-n-butylpyrazolidine
102. Ossifenbutazone [dcit]
103. Ab00052064_08
104. Dsstox_cid_25291
105. Dsstox_rid_80784
106. Dsstox_gsid_45291
107. Oxifenbutazona [inn-spanish]
108. Oxyphenbutazonum [inn-latin]
109. Ossifenbutazone
110. Opb
111. Ccris 6717
112. Hsdb 3144
113. Sr-05000001689
114. Einecs 204-936-2
115. 1-p-hydroxyphenyl-2-phenyl-3,5-dioxo-4-n-butylpyrazolidine
116. Nsc 526053
117. Brn 0307474
118. Metabolitie
119. Romaxin
120. Reozon
121. Unii-a7d84513gv
122. Ai3-26792
123. 3,5-pyrazolidinedione, 4-butyl-1-(4-hydroxyphenyl)-2-phenyl
124. Ncgc00016389-01
125. Cas-129-20-4
126. Reozon (tn)
127. Decanoylm-nitroaniline
128. Oxyphenbutazone (tn)
129. Spectrum_001073
130. Oxyphenbutazone, Tanderil
131. Prestwick0_001049
132. Prestwick1_001049
133. Prestwick2_001049
134. Prestwick3_001049
135. Spectrum2_000153
136. Spectrum3_000535
137. Spectrum4_001155
138. Spectrum5_000998
139. Chembl1228
140. Oxyphenbutazone [mi]
141. Schembl25857
142. Bspbio_000978
143. Bspbio_002149
144. Kbiogr_001729
145. Kbioss_001553
146. Divk1c_000302
147. Oxyphenbutazone [hsdb]
148. Spectrum1500455
149. Spbio_000286
150. Spbio_002909
151. Bpbio1_001076
152. Dtxsid1045291
153. Oxyphenbutazone [who-dd]
154. Hms500p04
155. Hy-b1355a
156. Kbio1_000302
157. Kbio2_001553
158. Kbio2_004121
159. Kbio2_006689
160. Kbio3_001649
161. Ninds_000302
162. 4-butyl-1-(4-hydroxyphenyl)-2-phenyl-pyrazolidine-3,5-dione
163. Bdbm200298
164. Hms1571a20
165. Hms1920d20
166. Hms2091l20
167. Hms2098a20
168. Hms2236g07
169. Hms3373b01
170. Hms3715a20
171. Pharmakon1600-01500455
172. Bcp20014
173. Tox21_110414
174. 3,5-pyrazolidinedione, 4-butyl-1-(p-hydroxyphenyl)-2-phenyl- (van)
175. Ccg-38944
176. Nsc526053
177. Nsc757261
178. Akos024362800
179. Tox21_110414_1
180. Db03585
181. Idi1_000302
182. (+/-)-oxyphenbutazone Anhydrous
183. Ncgc00094748-01
184. Ncgc00094748-02
185. Ncgc00094748-03
186. Ncgc00094748-04
187. Ncgc00094748-07
188. (+/-)-oxyphenbutazone-d9(n-butyl-d9)
189. Sbi-0051471.p003
190. Oxyphenbutazone Anhydrous, (+/-)-
191. 3, 4-butyl-1-(p-hydroxyphenyl)-2-phenyl-
192. Ab00052064
193. Cs-0013099
194. Ft-0673468
195. Ro-04-4410
196. Wln: T5vnnv Ehj Br Dq & Cr & E4
197. 3, 4-butyl-1-(4-hydroxyphenyl)-2-phenyl-
198. Oxyphenbutazone 4-hydroxyphenylbutazone
199. C19494
200. D08324
201. G-27202
202. G-29701
203. J-005659
204. Q4116192
205. Sr-05000001689-1
206. Sr-05000001689-3
207. Brd-a33749298-001-05-0
| Molecular Weight | 324.4 g/mol |
|---|---|
| Molecular Formula | C19H20N2O3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 324.14739250 g/mol |
| Monoisotopic Mass | 324.14739250 g/mol |
| Topological Polar Surface Area | 60.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 454 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal; Anti-Inflammatory Agents, Topical
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
EPISCLERITIS & UVEITIS ASSOC WITH RHEUMATOID ARTHRITIS HAVE SOMETIMES BEEN TREATED SUCCESSFULLY WITH...OXYPHENBUTAZONE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 972
...HAS MILD URICOSURIC EFFECT IN EXPTL ANIMALS & MAN, PROBABLY ATTRIBUTABLE TO ONE OF ITS METABOLITES.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 339
...HAS MILD URICOSURIC EFFECT IN EXPTL ANIMALS & MAN... URICOSURIC EFFECT RESULTS FROM DIMINISHED TUBULAR REABSORPTION OF URIC ACID. ...CAUSES SIGNIFICANT RETENTION OF SODIUM & CHLORIDE, ACCOMPANIED BY REDN IN URINE VOL... REDUCES UPTAKE OF IODINE BY THYROID GLAND...ALSO INHIBITS ENZYMES OF KREBS CYCLE... /PHENYLBUTAZONE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 339
For more Therapeutic Uses (Complete) data for OXYPHENBUTAZONE (7 total), please visit the HSDB record page.
...40-YR OLD MAN RECEIVED TOTAL DOSE OF 1 G OXYPHENBUTAZONE OVER APPROX 5 DAYS, 2 MO BEFORE ADMISSION TO HOSPITAL WITH SEVERE ANEMIA & LEUCOCYTOSIS; HE DIED ON THIRD DAY AFTER DEVELOPING THIS CONDITION.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 194
...WHITE-BLOOD CELL DISORDER CONCERNED 47-YR-OLD MAN WHO, 13 MO BEFORE ADMISSION WITH TEMP, HAD BEEN GIVEN 1.9 G OXYPHENBUTAZONE OVER 2-WK PERIOD. ON ADMISSION, HE WAS GIVEN 0.6 G OF DRUG; BLOOD EXAM SHOWED THAT HE HAD LEUKEMIA, OF WHICH HE DIED APPROX 4 MO LATER.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 194
IT SHOULD ALWAYS BE TAKEN IMMEDIATELY AFTER MEALS OR WITH FULL GLASS OF MILK, TO MINIMIZE GASTRIC IRRITATION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1051
OXYPHENBUTAZONE IS SAID TO CAUSE SOMEWHAT LESS GASTRIC IRRITATION /THAN PHENYLBUTAZONE/. ... IT SHOULD BE TAKEN IN 3 OR 4 DIVIDED PORTIONS AFTER MEALS TO LESSEN GASTRIC IRRITATION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 341
For more Drug Warnings (Complete) data for OXYPHENBUTAZONE (8 total), please visit the HSDB record page.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AA - Butylpyrazolidines
M01AA03 - Oxyphenbutazone
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA04 - Oxyphenbutazone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC02 - Oxyphenbutazone
...OXYPHENBUTAZONE IS EXTENSIVELY BOUND TO PLASMA PROTEINS & HAS PLASMA HALF-TIME OF SEVERAL DAYS. ...ONLY SLOWLY EXCRETED IN URINE, SINCE BINDING TO PLASMA PROTEIN LIMITS...GLOMERULAR FILTRATION, &...RELATIVELY HIGH PKA, WHICH FAVORS PASSIVE REABSORPTION IN DISTAL TUBULE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 340
OXIDN OF PHENYLBUTAZONE DURING CHRONIC DOSING BY MEASURING URINARY EXCRETION OF 3 METABOLITES, OXYPHENBUTAZONE GAMMA-HYDROXYPHENBUTAZONE & P,GAMMA-HYDROXYPHENBUTAZONE DURING INFUSION OF HYDROCORTISONE SODIUM SUCCINATE IS REPORTED.
PMID:911609 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1429144 AARBAKKE J ET AL; BR J CLIN PHARMACOL 4: 621-622 (1977)
IN MAN, OXYPHENBUTAZONE HAS BIOLOGICAL HALF-LIFE OF ABOUT 2 DAYS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 190
...HAS PROMINENT ANTI-INFLAMMATORY EFFECTS IN ANIMALS, & COMPARABLE EFFECTS... IN PT WITH RHEUMATOID ARTHRITIS & RELATED DISORDERS. ...INHIBITS BIOSYNTHESIS OF PROSTAGLANDINS, UNCOUPLES OXIDATIVE PHOSPHORYLATION, & INHIBITS ATP-DEPENDENT BIOSYNTHESIS OF MUCOPOLYSACCHARIDE SULFATES IN CARTILAGE. /PHENYLBUTAZONE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 339