

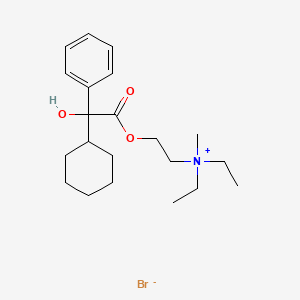

1. 2-((cyclohexylhydroxyphenylacetyl)oxy)-n,n-diethyl-n-methylethanaminium
2. Atrenyl
3. Bromide, Oxyphenonium
4. Chloride, Oxyphenonium
5. Iodide, Oxyphenonium
6. Metacin
7. Methacin
8. Oxyphenon
9. Oxyphenonium
10. Oxyphenonium Bromide, (+)-isomer
11. Oxyphenonium Bromide, (+-)-isomer
12. Oxyphenonium Bromide, (-)-isomer
13. Oxyphenonium Chloride
14. Oxyphenonium Iodide
15. Oxyphenonium Iodide, (r)-isomer
16. Oxyphenonium Iodide, (s)-isomer
17. Oxyphenonium, (+-)-isomer
18. Oxyphenonium, (r)-isomer
19. Oxyphenonium, (s)-isomer
20. Spastrex
1. 50-10-2
2. Spasmophen
3. Oxifenon
4. Oxyfenon
5. Oxyphenon
6. Antrenyl
7. Oxyphenoniumbromide
8. Antrenil
9. Spasmodin
10. Ba-5473
11. Oxyphenonium (bromide)
12. Nsc-759248
13. Mls000028620
14. S9421hwb3z
15. 2-(2-cyclohexyl-2-hydroxy-2-phenylacetyl)oxyethyl-diethyl-methylazanium;bromide
16. Smr000058660
17. Subranyl
18. Antrenyl Bromide
19. C-5473
20. Oxyphenonium Bromide (inn)
21. Dsstox_cid_25632
22. Dsstox_rid_81014
23. Ethanaminium, 2-((cyclohexylhydroxyphenylacetyl)oxy)-n,n-diethyl-n-methyl-, Bromide
24. Dsstox_gsid_45632
25. Oxyphenonium Bromide [inn]
26. Cas-50-10-2
27. Oxyphenonii Bromidum
28. Bromuro De Oxifenonio
29. Bromure D'oxyphenonium
30. Oxyphenonium Bromide [inn:ban]
31. Oxyphenonii Bromidum [inn-latin]
32. Ncgc00018256-02
33. Einecs 200-010-7
34. Bromure D'oxyphenonium [inn-french]
35. Bromuro De Oxifenonio [inn-spanish]
36. Unii-s9421hwb3z
37. C 5473
38. Antrenyl (tn)
39. Opera_id_1635
40. Diethyl(2-hydroxyethyl)methylammonium Bromide Alpha-phenylcyclohexaneglycolate
41. Regid_for_cid_5748
42. Mls001076268
43. Mls001424168
44. Schembl248890
45. Chembl1200906
46. Dtxsid4045632
47. Oxyphenonium Bromide [mi]
48. Hms2052o07
49. Hms2230j18
50. Hms3371m04
51. Hms3394o07
52. Pharmakon1600-01505783
53. (+/-)-oxyphenonium Bromide
54. Oxyphenonium Bromide [vandf]
55. Tox21_110851
56. Nsc759248
57. Oxyphenonium Bromide [mart.]
58. Oxyphenonium Bromide [who-dd]
59. Akos030507607
60. Tox21_110851_1
61. Ccg-101094
62. Ccg-213986
63. Nc00344
64. Ammonium, Diethyl(2-hydroxyethyl)methyl-, Bromide, Alpha-phenylcyclohexaneglycolate
65. Ncgc00018256-06
66. Oxyphenonium Bromide [orange Book]
67. D06877
68. Sr-01000003140
69. Q7116109
70. Sr-01000003140-4
71. Z1551429731
72. {2-[(2-cyclohexyl-2-hydroxy-2-phenylacetyl)oxy]ethyl}diethylmethylazanium Bromide
73. 2-(2-cyclohexyl-2-hydroxy-2-phenylacetoxy)-n,n-diethyl-n-methylethan-1-aminium Bromide
74. Diethyl(2-hydroxyethyl)methylammonium Bromide .alpha.-phenylcyclohexaneglycolate
75. Ethanaminium, 2-((2-cyclohexyl-2-hydroxy-2-phenylacetyl)oxy)-n,n-diethyl-n-methyl-, Bromide (1:1)
| Molecular Weight | 428.4 g/mol |
|---|---|
| Molecular Formula | C21H34BrNO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 427.17221 g/mol |
| Monoisotopic Mass | 427.17221 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 409 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)