

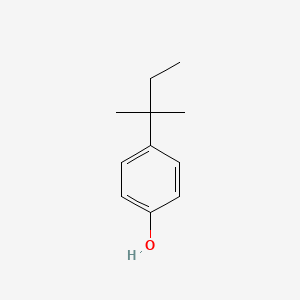

1. 4-tert-pentyphenol
2. P-tert-amylphenol
3. P-tert-amylphenol, Monopotassium Salt
4. P-tert-amylphenol, Monosodium Salt
5. Para-tertiary-amyl-phenol
1. 80-46-6
2. 4-tert-pentylphenol
3. P-tert-pentylphenol
4. P-tert-amylphenol
5. 4-t-amylphenol
6. 4-(2-methylbutan-2-yl)phenol
7. Pentaphen
8. 4-(1,1-dimethylpropyl)phenol
9. Phenol, 4-(1,1-dimethylpropyl)-
10. Amilphenol
11. Amilfenol
12. Ptap
13. Amyl Phenol 4t
14. Phenol, P-tert-pentyl-
15. Tert-amylphenol
16. P-(1,1-dimethylpropyl)phenol
17. Ucar Amyl Phenol 4t
18. P-t-pentylphenol
19. 4-(tert-pentyl)phenol
20. 2-methyl-2-p-hydroxyphenylbutane
21. 4-(1,1-dimethylpropyl)-1-phenol
22. 1-hydroxy-4-(1,1-dimethylpropyl)benzene
23. 4-t-pentylphenol
24. Phenol, P-(tert-pentyl)-
25. Mfcd00002369
26. Nsc 403672
27. 6np9lyk846
28. Chembl195693
29. Chebi:35096
30. Nsc-403672
31. Ncgc00091655-02
32. P-(.alpha.,.alpha.-dimethylpropyl)phenol
33. Dsstox_cid_1771
34. Phenol,1-dimethylpropyl)-
35. Dsstox_rid_76317
36. Dsstox_gsid_21771
37. Para-tert-amylphenol
38. Caswell No. 050
39. Wln: Qr D1x1&1&1
40. Cas-80-46-6
41. Ccris 4693
42. P-(tert-amyl)phenol
43. Hsdb 5236
44. Einecs 201-280-9
45. P-(alpha,alpha-dimethylpropyl)phenol
46. Epa Pesticide Chemical Code 064101
47. Amylphenol, P-tert-
48. Brn 1908224
49. Unii-6np9lyk846
50. P-tertamylphenol
51. Ai3-00460
52. P-t-amyl Phenol
53. Nipacide Ptap
54. Pentaphen 67
55. Para-tertiary Amylphenol
56. 4-tert-amylphenol, 99%
57. Ec 201-280-9
58. Amylphenol [mart.]
59. Schembl49704
60. 4-06-00-03383 (beilstein Handbook Reference)
61. Mls002152935
62. Bidd:er0210
63. Dtxsid8021771
64. Nrzwynltfldqqx-uhfffaoysa-
65. Nsc4965
66. P-tert-pentylphenol [mi]
67. P-(1,1-dimethyl Propyl) Phenol
68. 4-(1,1-dimethyl-propyl)-phenol
69. Hms3039m10
70. Nsc-4965
71. Zinc1680640
72. Tox21_111159
73. Tox21_202351
74. Tox21_300088
75. Bdbm50410536
76. Nsc403672
77. Akos000119604
78. Tox21_111159_1
79. 4-tert-amylphenol, Analytical Standard
80. 1-hydroxy-4-(2-methyl-2-butyl)benzene
81. Ncgc00091655-01
82. Ncgc00091655-03
83. Ncgc00091655-04
84. Ncgc00091655-05
85. Ncgc00254041-01
86. Ncgc00259900-01
87. Ac-16506
88. Smr001224530
89. Db-000247
90. A0460
91. Am20041188
92. Cs-0152629
93. Ft-0704191
94. 4-(1,1-dimethylpropyl)phenol [hsdb]
95. A24574
96. E76129
97. P-(1,1-dimethylpropyl)phenol;para-tert-amylphenol
98. W-109280
99. Q26840951
100. Z1262246132
| Molecular Weight | 164.24 g/mol |
|---|---|
| Molecular Formula | C11H16O |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 164.120115130 g/mol |
| Monoisotopic Mass | 164.120115130 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 132 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
3(?). 3 = moderately toxic: probable oral lethal dose (human) 0.5-5 g/kg, between 1 oz and 1 pint for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-189
Assoc of (14)C-labelled p-tert-amylphenol with human serum and bacterial Micrococcus lysodeikticus proteins was most clear-cut among phenolic deriv. Protein binding could explain interference of serum with germicidal effects of phenolic disinfectants.
PMID:5657532 Starr JE et al; J Pharm Sci 57 (5): 768 (1968)
Yields 4-tert-amylphenyl-beta-d-glucuronide in rabbits; Jellinick, PH, Biochem J, 58, 262 (1954). /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. A-55