

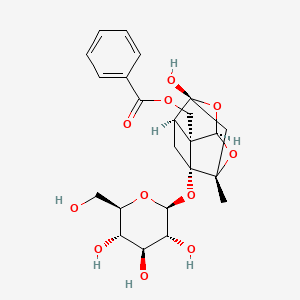

1. Peoniflorin
2. Peoniflorin Sulfonate
1. 23180-57-6
2. Peoniflorin
3. Paeonia Moutan
4. Paeony Root
5. Nsc 178886
6. 21aiq4ev64
7. ((2s,2ar,2a1s,3ar,4r,5ar)-4-hydroxy-2-methyl-2a-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexahydro-2h-1,5-dioxa-2,4-methanocyclobuta[cd]pentalen-2a1-yl)methyl Benzoate
8. Unii-21aiq4ev64
9. Ccris 6494
10. Nsc178886
11. Nsc-178886
12. .beta.-d-glucopyranoside, (1ar,2s,3ar,5r,5ar,5bs)-5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl
13. .beta.-d-glucopyranoside, (1ar,2s,3ar,5r,5ar,5bs)-5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta[cd]pentalen-1a(2h)-yl
14. Einecs 245-476-2
15. Mfcd00869331
16. Peoniflorin [inci]
17. Paeoniflorin [usp-rs]
18. Schembl549033
19. Chebi:7889
20. Chembl4303209
21. Dtxsid2042648
22. Paeoniflorin, Analytical Standard
23. Paeoniflorin, >=98% (hplc)
24. Hms3884d17
25. Hy-n0293
26. Zinc8234328
27. S2410
28. Akos025311455
29. Ccg-269549
30. As-12193
31. Beta-d-glucopyranoside, (1as,2r,3ar,5r,5ar,5bs)-5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl
32. C09959
33. Ab01566855_01
34. 180p576
35. Q-100296
36. Q7124104
37. ((2s,2ar,2a1s,3ar,4r,5ar)-4-hydroxy-2-methyl-2a-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexahydro-2h-1,5-dioxa-2,4-methanocyclobuta[cd]pentalen-2a1-yl)methylbenzoate
38. .beta.-d-glucopyranoside, 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl, (1ar-(1a.alpha.,2.beta.,3a.alpha.,5.alpha.,5a.alpha.,5b.alpha.))-
39. [(1r,2s,3r,5r,6r,8s)-6-hydroxy-8-methyl-3-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9,10-dioxatetracyclo[4.3.1.02,5.03,8]decan-2-yl]methyl Benzoate
40. [hydroxy-methyl-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-[?]yl]methyl Benzoate
41. 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-lh-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl-beta-d-glucopyranoside
42. B-d-glucopyranoside,(1ar,2s,3ar,5r,5ar,5bs)-5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta[cd]pentalen-1a(2h)-yl
43. Beta-d-glucopyranoside, 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl, (1ar-(1a-alpha,2-beta,3a-alpha,5-alpha,5a-alpha,5b-alpha))-
44. Beta-d-glucopyranoside, 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1h-3,4-dioxacyclobuta(cd)pentalen-1a(2h)-yl, (1ar-(1aalpha,2beta,3aalpha,5alpha,5aalpha,5balpha))-
| Molecular Weight | 480.5 g/mol |
|---|---|
| Molecular Formula | C23H28O11 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 480.16316171 g/mol |
| Monoisotopic Mass | 480.16316171 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 849 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)