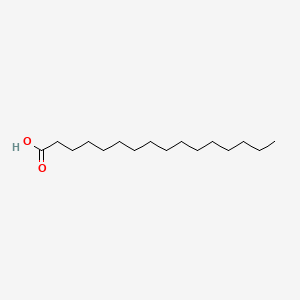

1. Acid, Hexadecanoic
2. Acid, Palmitic
3. Calcium Palmitate
4. Hexadecanoic Acid
5. Palmitate, Calcium
6. Palmitate, Sodium
7. Sodium Palmitate
1. Hexadecanoic Acid
2. 57-10-3
3. Cetylic Acid
4. Palmitate
5. N-hexadecanoic Acid
6. Hexadecylic Acid
7. Hydrofol
8. N-hexadecoic Acid
9. 1-pentadecanecarboxylic Acid
10. Palmitinic Acid
11. Pentadecanecarboxylic Acid
12. C16 Fatty Acid
13. Hexadecanoate
14. Hexaectylic Acid
15. 1-hexyldecanoic Acid
16. Hexadecoic Acid
17. Industrene 4516
18. Emersol 140
19. Emersol 143
20. Hystrene 8016
21. Hystrene 9016
22. Palmitinsaeure
23. Palmitic Acid, Pure
24. Fema No. 2832
25. Palmitic Acid 95%
26. Kortacid 1698
27. Loxiol Ep 278
28. Palmitic Acid (natural)
29. Hydrofol Acid 1690
30. Prifac 2960
31. Fatty Acids, C14-18
32. Pristerene 4934
33. Edenor C16
34. Lunac P 95kc
35. C16:0
36. Lunac P 95
37. Lunac P 98
38. Cetyl Acid
39. Hsdb 5001
40. Ai3-01594
41. Nsc 5030
42. Pristerene-4934
43. Palmitic Acid (nf)
44. Glycon P-45
45. Chebi:15756
46. Nsc5030
47. Prifac-2960
48. Nsc-5030
49. Hexadecanoic Acid (9ci)
50. Mfcd00002747
51. Palmitic Acid (7ci,8ci)
52. Chembl82293
53. Ch3-[ch2]14-cooh
54. Imex C 1498
55. 2v16eo95h1
56. N-hexadecoate
57. Lmfa01010001
58. Pa 900
59. 67701-02-4
60. Fa 16:0
61. Fa 1695
62. 1-hexyldecanoate
63. Ncgc00164358-01
64. Dsstox_cid_1602
65. Pentadecanecarboxylate
66. Hexadecanoic Acid 10 Microg/ml In Acetonitrile
67. Dsstox_rid_76229
68. Dsstox_gsid_21602
69. C16h32o2
70. Plm
71. Palmic Acid
72. Hexadecanoate (n-c16:0)
73. Cas-57-10-3
74. Ccris 5443
75. Sr-01000944716
76. Einecs 200-312-9
77. Palmitic Acid [usan:nf]
78. Brn 0607489
79. Palmitoate
80. Hexadecoate
81. Palmitinate
82. Palmitic-acid
83. Palmitoic Acid
84. Hexadecanoicacid
85. Aethalic Acid
86. Unii-2v16eo95h1
87. Hexadecanoic Acid Palmitic Acid
88. 2hmb
89. 2hnx
90. Fatty Acid Pathway
91. Palmitic Acid_jeyam
92. Palmitic Acid, Fcc
93. Kortacid 1695
94. Palmitic Acid_ragusa
95. Univol U332
96. Prifrac 2960
97. Hexadecanoic Acid Anion
98. 3v2q
99. Palmitic Acid, >=99%
100. Bmse000590
101. Epitope Id:141181
102. Ec 200-312-9
103. Cetyl Acid [vandf]
104. Palmitic Acid [ii]
105. Palmitic Acid [mi]
106. Schembl6177
107. Palmitic Acid [dsc]
108. Palmitic Acid [fcc]
109. Palmitic Acid [fhfi]
110. Palmitic Acid [hsdb]
111. Palmitic Acid [inci]
112. Palmitic Acid [usan]
113. 4-02-00-01157 (beilstein Handbook Reference)
114. Fat
115. Wln: Qv15
116. P5585_sigma
117. Palmitic Acid [vandf]
118. Palmitic Acid [mart.]
119. Gtpl1055
120. Qspl 166
121. Palmitic Acid [usp-rs]
122. Palmitic Acid [who-dd]
123. (1(1)(3)c)hexadecanoic Acid
124. Dtxsid2021602
125. 1b56
126. Hms3649n08
127. Palmitic Acid, Analytical Standard
128. Palmitic Acid, Bioxtra, >=99%
129. Palmitic Acid, Grade Ii, ~95%
130. Hy-n0830
131. Palmitic Acid, Natural, 98%, Fg
132. Zinc6072466
133. Tox21_112105
134. Tox21_201671
135. Tox21_302966
136. Ac9381
137. Bbl011563
138. Bdbm50152850
139. Palmitic Acid [ep Monograph]
140. S3794
141. Stl146733
142. Edenor C 16-98-100
143. Palmitic Acid, >=95%, Fcc, Fg
144. Akos005720983
145. Tox21_112105_1
146. Ccg-267027
147. Cr-0047
148. Db03796
149. Palmitic Acid, For Synthesis, 98.0%
150. Surfaxin Component Palmitic Acid
151. Ncgc00164358-02
152. Ncgc00164358-03
153. Ncgc00256424-01
154. Ncgc00259220-01
155. Bp-27917
156. Lucinactant Component Palmitic Acid
157. Palmitic Acid, Purum, >=98.0% (gc)
158. Sy006518
159. Cs-0009861
160. Ft-0626965
161. Ft-0772579
162. N2456
163. P0002
164. P1145
165. Palmitic Acid, Saj First Grade, >=95.0%
166. A14813
167. C00249
168. D05341
169. Palmitic Acid, Vetec(tm) Reagent Grade, 98%
170. Palmitic Acid, >=98% Palmitic Acid Basis (gc)
171. A831313
172. Hexadecanoic Acid-13c16 (algal Source) (
173. Q209727
174. Sr-01000944716-1
175. Sr-01000944716-2
176. Ba71c79b-c9b1-451a-a5be-b480b5cc7d0c
177. Palmitic Acid (constituent Of Spirulina) [dsc]
178. F0001-1488
179. Z955123552
180. Palmitic Acid (constituent Of Flax Seed Oil) [dsc]
181. Palmitic Acid (constituent Of Saw Palmetto) [dsc]
182. Palmitic Acid, Certified Reference Material, Tracecert(r)
183. Palmitic Acid (constituent Of Borage Seed Oil) [dsc]
184. Palmitic Acid, European Pharmacopoeia (ep) Reference Standard
185. Palmitic Acid (constituent Of Evening Primrose Oil) [dsc]
186. Palmitic Acid, United States Pharmacopeia (usp) Reference Standard
187. Palmitic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
188. Sodium Palmitate, Palmitic Acid Sodium Salt, Sodium Hexadecanoate, Sodium Pentadecanecarboxylate, Hsdb 759
| Molecular Weight | 256.42 g/mol |
|---|---|
| Molecular Formula | C16H32O2 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 14 |
| Exact Mass | 256.240230259 g/mol |
| Monoisotopic Mass | 256.240230259 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 178 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Recent studies indicate that lipid metabolic changes affect the survival of multiple myeloma (MM) cells. Time-of-flight secondary ion mass spectrometry (TOF-SIMS), an imaging mass spectrometry technique, is used to visualize the subcellular distribution of biomolecules including lipids. We therefore applied this method to human clinical specimens to analyze the membrane fatty acid composition and determine candidate molecules for MM therapies. We isolated MM cells and normal plasma cells (PCs) from bone marrow aspirates of MM patients and healthy volunteers, respectively, and these separated cells were analyzed by TOF-SIMS. Multiple ions including fatty acids were detected and their ion counts were estimated. In MM cells, the mean intensity of palmitic acid was significantly lower than the mean intensity in PCs. In a cell death assay, palmitic acid reduced U266 cell viability dose-dependently at doses between 50 and 1000 uM. The percentage of apoptotic cells increased from 24 hr after palmitic acid administration. In contrast, palmitic acid had no effect on the viability of normal peripheral blood mononuclear cells (PBMCs). The results of this study indicated that palmitic acid is a potential candidate for novel therapeutic agents that specifically attack MM cells.
PMID:25846050 Nagata Y et al; Leuk Res 39 (6): 638-45 (2015)
/EXPL THER/ Approximately 80% of all new HIV-1 infections are acquired through sexual contact. Currently, there is no clinically approved microbicide, indicating a clear and urgent therapeutic need. We recently reported that palmitic acid (PA) is a novel and specific inhibitor of HIV-1 fusion and entry. Mechanistically, PA inhibits HIV-1 infection by binding to a novel pocket on the CD4 receptor and blocks efficient gp120-to-CD4 attachment. Here, we wanted to assess the ability of PA to inhibit HIV-1 infection in cervical tissue ex vivo model of human vagina, and determine its effect on Lactobacillus (L) species of probiotic vaginal flora. Our results show that treatment with 100-200 uM PA inhibited HIV-1 infection in cervical tissue by up to 50%, and this treatment was not toxic to the tissue or to L. crispatus and jensenii species of vaginal flora. In vitro, in a cell free system that is independent of in vivo cell associated CD4 receptor; we determined inhibition constant (Ki) to be ~2.53 uM. These results demonstrate utility of PA as a model molecule for further preclinical development of a safe and potent HIV-1 entry microbicide inhibitor.
PMID:21949756 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176227 Lin X et al; PLoS One 6 (9): e24803 (2011)
/EXPL THER/ In a recent laboratory study, a fatty acid from seaweed reduced the ability of HIV-1 viruses to enter immune system cells. The study was reported in the journal AIDS Research and Human Retroviruses. Drug-resistant strains of HIV-1 have been on the rise, prompting the need for new therapeutic agents. Previous studies have demonstrated that products derived from natural sources have the potential to inhibit HIV-1 infection. In this laboratory study, researchers evaluated palmitic acid (from Sargassum fusiforme, a type of seaweed that grows off the coasts of Japan and China) to see if palmitic acid reduced the ability of HIV-1 viruses to enter CD4+ T-cells (white blood cells that are HIV-1's main target). Palmitic acid blocked both X4-tropic and R5-tropic viruses, the HIV viruses that use a particular receptor (X4 or R5) to enter a cell. In addition, the study's findings showed that palmitic acid protected other cells against HIV-1, reducing X4 infection in primary peripheral blood lymphocytes and R5 infection in primary macrophages (white blood cells). In all cases, the extent of the blocking effect depended on the concentration of palmitic acid, and most cells remained viable (alive) after treatment. The researchers noted that understanding the relationship between palmitic acid and CD4 may lead to development of an effective microbicide product for preventing sexual transmission of HIV.
NIH; National Center for Complimentary and Integrative Health; Laboratory Study Explores Anti-HIV Potential of Palmitic Acid (January, 2012); Available from, as of October 18, 2017: https://nccih.nih.gov/research/results/spotlight/121409.htm
/EXPL THER/ The high rate of HIV-1 mutation and the frequent sexual transmission highlight the need for novel therapeutic modalities with broad activity against both CXCR4 (X4) and CCR5 (R5)-tropic viruses. We investigated a large number of natural products, and from Sargassum fusiforme we isolated and identified palmitic acid (PA) as a natural small bioactive molecule with activity against HIV-1 infection. Treatment with 100 uM PA inhibited both X4 and R5 independent infection in the T cell line up to 70%. Treatment with 22 uM PA inhibited X4 infection in primary peripheral blood lymphocytes (PBL) up to 95% and 100 uM PA inhibited R5 infection in primary macrophages by over 90%. Inhibition of infection was concentration dependent, and cell viability for all treatments tested remained above 80%, similar to treatment with 10(-6)M nucleoside analogue 2',3'-dideoxycytidine (ddC). Micromolar PA concentrations also inhibited cell-to-cell fusion and specific virus-to-cell fusion up to 62%. PA treatment did not result in internalization of the cell surface CD4 receptor or lipid raft disruption, and it did not inhibit intracellular virus replication. PA directly inhibited gp120-CD4 complex formation in a dose-dependent manner. We used fluorescence spectroscopy to determine that PA binds to the CD4 receptor with K(d) approximately 1.5 +/- 0.2 uM, and we used one-dimensional saturation transfer difference NMR (STD-NMR) to determined that the PA binding epitope for CD4 consists of the hydrophobic methyl and methelene groups located away from the PA carboxyl terminal, which blocks efficient gp120-CD4 attachment. These findings introduce a novel class of antiviral compound that binds directly to the CD4 receptor, blocking HIV-1 entry and infection. Understanding the structure-affinity relationship (SAR) between PA and CD4 should lead to the development of PA analogs with greater potency against HIV-1 entry.
PMID:20001317 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828184 Lee DY et al; AIDS Res Hum Retroviruses 25 (12): 1231-41 (2009)
Palmitic acid is the first fatty acid produced during lipogenesis (fatty acid synthesis) and from which longer fatty acids can be produced. Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC) which is responsible for converting acetyl-ACP to malonyl-ACP on the growing acyl chain, thus preventing further palmitate generation
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Added (14)C-labeled palmitate was more significantly incorporated into lipid fractions of muscle fibers from fetal and neonatal monkeys than those from adults. /Palmitate/
Beatty CH, Bocek RM; Amer J Physiol 219 (5): 1311 (1970)
More (14)C-labeled palmitate was incorporated into lipid by adipose tissue of genetically obese rats than by controls. /Palmitate/
PMID:5506400 Bray et al; Metab Clin Exp 19 (10): 839 (1970)
Radioactivity has been traced to the heart, liver, lung, spleen, kidney, muscle, intestine, adrenal, blood, and lymph, and adipose, mucosal, and dental tissues after administration of radioactive oleic, palmitic, or stearic acids.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p. 341 J Am Col Toxicol 6 (3): 321-401 (1987). Available from, as of November 22, 2017: https://www.cir-safety.org/ingredients
Fatty acids originating from adipose tissue stores are either bound to serum albumin or remain unesterified in the blood.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p. 341 J Am Col Toxicol 6 (3): 321-401 (1987). Available from, as of November 22, 2017: https://www.cir-safety.org/ingredients
For more Absorption, Distribution and Excretion (Complete) data for Palmitic acid (7 total), please visit the HSDB record page.
Palmitic acid is rapidly metabolized, primarily by beta-oxidation. In addition to oxidative breakdown, palmitic acid undergoes a variety of conversion reactions in the liver and intestinal mucosa to stearic, oleic, palmitoleic, and myristic acids. omega-Oxidation, prior to beta-oxidation, may account for 5 to 10% of the hepatic metabolism of palmitic acid in the starved rat. After oxidation or conversion to other long-chain fatty acids or phospholipids, the carbon skeleton of palmitic acid is stored in the form of esterified cholesterol or returned to the plasma, depending upon the nutritional state of the organism.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 743
Proposed mechanisms for fatty acid uptake by different tissues range from passive diffusion to facilitated diffusion or a combination of both. Fatty acids taken up by the tissues can either be stored in the form of triglycerides (98% of which occurs in adipose tissue depots) or they can be oxidized for energy via the beta-oxidation and tricarboxylic acid cycle pathways of catabolism. /Fatty acids/
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p. 341 J Am Col Toxicol 6 (3): 321-401 (1987). Available from, as of November 22, 2017: https://www.cir-safety.org/ingredients
The beta-oxidation of fatty acids occurs in most vertebrate tissues (except the brain) using an enzyme complex for the series of oxidation and hydration reactions resulting in the cleavage of acetate groups as acetyl-CoA (coenzyme A). An additional isomerization reaction is required for the complete catabolism of oleic acid. Alternate oxidation pathways can be found in the liver (omega-oxidation) and in the brain (alpha-oxidation). /fatty acids/
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p. 341 J Am Col Toxicol 6 (3): 321-401 (1987). November 22, 2017: https://www.cir-safety.org/ingredients
Fatty acid biosynthesis from acetyl-CoA takes place primarily in the liver, adipose tissue, and mammary glands of higher animals. Successive reduction and dehydration reactions yield saturated fatty acids up to a 16-carbon chain length. /Fatty acids/
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Oleic Acid, Lauric Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. p. 341 J Am Col Toxicol 6 (3): 321-401 (1987). Available from, as of November 22, 2017: https://www.cir-safety.org/ingredients
Palmitic acid has known human metabolites that include 15-Hydroxy-hexadecanoic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... Excessive palmitoylcarnitine formation and exhausted L-carnitine stores leading to energy depletion, attenuated acetylcholine synthesis and oxidative stress to be main mechanisms behind PA-induced neuronal loss.High PA exposure is suggested to be a factor in causing diabetic neuropathy and gastrointestinal dysregulation.
PMID:24312551 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3849255 Voss U et al; PLoS One 8 (12): e81413 (2013)
... First phase insulin release response was lost in these islets. FFAs slightly increased the insulin output of normal fresh pancreas beta-cells. However, chronic exposure to FFAs resulted in loss of first phase insulin release and blunted insulin secretion response to various levels of D-glucose stimulation.
PMID:12688627 Ayvaz G et al; Diabetes Metab 28 (6 Pt 2): 3S7-12 (2002)