

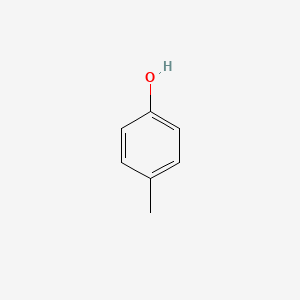

1. 4-cresol
2. 4-cresol, Aluminum Salt
3. 4-cresol, Potassium Salt
4. 4-cresol, Sodium Salt
5. 4-methylphenol
6. Para-cresol
1. 4-methylphenol
2. 106-44-5
3. 4-cresol
4. 4-hydroxytoluene
5. Phenol, 4-methyl-
6. P-methylphenol
7. Para-cresol
8. P-hydroxytoluene
9. P-tolyl Alcohol
10. P-kresol
11. P-oxytoluene
12. P-toluol
13. P-cresylic Acid
14. 1-hydroxy-4-methylbenzene
15. Paracresol
16. P-methylhydroxybenzene
17. 1-methyl-4-hydroxybenzene
18. Paramethyl Phenol
19. Para-cresylic Acid
20. 4-methyl Phenol
21. Cresol, Para-
22. Cresol, P-
23. P-kresol [german]
24. Cresol, P-isomer
25. Fema No. 2337
26. Cresol, Para
27. P-methyl Phenol
28. 4-methyl-phenol
29. 4-methylphenol (p-cresol)
30. Nsc 3696
31. Mfcd00002376
32. 1mxy2um8nv
33. Chembl16645
34. Phenol, 4-methyl-, Homopolymer
35. Chebi:17847
36. Nsc-3696
37. Ncgc00091519-04
38. Toluene,4-hydroxy (para-cresol)
39. P-cresol [un2076] [poison, Corrosive]
40. Dsstox_cid_1869
41. Dsstox_rid_76375
42. Dsstox_gsid_21869
43. 27289-34-5
44. Cas-106-44-5
45. Ccris 647
46. Hsdb 1814
47. Einecs 203-398-6
48. Unii-1mxy2um8nv
49. Para Cresol
50. P-cresylate
51. P-methyl-phenol
52. Ai3-00150
53. 4 -methylphenol
54. Cresol,p-
55. Para-cresyl Alcohol
56. Phenol, 4-methyi
57. P-cresol, 99%
58. Spectrum_000850
59. P-cresol, High Purity
60. 4-methyl Phenol 99%
61. P-cresol [fhfi]
62. P-cresol [hsdb]
63. P-cresol [inci]
64. Spectrum2_000765
65. Spectrum4_001740
66. Spectrum5_000540
67. P-cresol [mi]
68. Schembl375
69. Bmse000458
70. Ec 203-398-6
71. Dsstox_rid_77380
72. Dsstox_rid_77554
73. Nciopen2_001516
74. Wln: Qr D1
75. Dsstox_gsid_24364
76. Dsstox_gsid_24858
77. Kbiogr_002160
78. Kbioss_001330
79. 1-hydroxyl 4-methyl Benzene
80. P-cresol, Analytical Standard
81. Bidd:er0010
82. Divk1c_000381
83. Spectrum1500209
84. P-cresol, >=99%, Fg
85. Spbio_000810
86. Schembl7812506
87. Sgcut00097
88. Dtxsid7021869
89. Hms501d03
90. Kbio1_000381
91. Kbio2_001330
92. Kbio2_003898
93. Kbio2_006466
94. Nsc3696
95. Paracresol [usp Impurity]
96. Ninds_000381
97. Hms1920a16
98. Hms2091i04
99. Pharmakon1600-01500209
100. Zinc897142
101. P-cresol, For Synthesis, 98.0%
102. 4-methylphenol, Analytical Standard
103. Nsc95259
104. To_000033
105. Tox21_113240
106. Tox21_113445
107. Tox21_200402
108. Tox21_201115
109. Tox21_300029
110. Bdbm50008543
111. Ccg-38990
112. Nsc-95259
113. Nsc756709
114. Stl183323
115. Akos000119005
116. Tox21_113445_1
117. Db01688
118. Idi1_000381
119. Ncgc00013272-01
120. Ncgc00091519-01
121. Ncgc00091519-02
122. Ncgc00091519-03
123. Ncgc00091519-05
124. Ncgc00091519-06
125. Ncgc00091519-07
126. Ncgc00091519-09
127. Ncgc00253980-01
128. Ncgc00257956-01
129. Ncgc00258667-01
130. 4-methylphenol 10 Microg/ml In Methanol
131. Ps-11958
132. 4-methylphenol 100 Microg/ml In Methanol
133. Cas-1319-77-3
134. P-cresol, Jis Special Grade, >=99.0%
135. Sbi-0051322.p003
136. Metacresol Impurity C [ep Impurity]
137. Ft-0660000
138. 4-methylphenol 100 Microg/ml In Cyclohexane
139. P-cresol, Puriss. P.a., >=99.0% (gc)
140. Amylmetacresol Impurity D [ep Impurity]
141. C01468
142. Ab00051955_02
143. Q312251
144. Sr-05000002037
145. J-001591
146. J-515803
147. Sr-05000002037-1
148. F1908-0066
149. 2876-02-0
| Molecular Weight | 108.14 g/mol |
|---|---|
| Molecular Formula | C7H8O |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 108.057514874 g/mol |
| Monoisotopic Mass | 108.057514874 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 62.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): Local antiseptic, parasiticide, disinfectant; has been used as an intestinal antiseptic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 460
Disinfectant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 460
Humans normally excrete approximately 50 mg of p-cresol in the urine daily. p-Cresol is produced endogenously from tyrosine by anaerobic bacteria in the gastrointestinal tract.
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 1259
The normal human excretes 16 to 39 mg p-cresol/day.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 446
Cresols are slightly more corrosive /to the skin or eyes/ than phenol, but systemic effects may be a little milder because of slower absorption.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-192
Healthy humans excrete an average of about 50 mg (range 16-74 mg) of p-cresol in the urine daily. Endogenous p-cresol is produced from tyrosine, an amino acid present in most proteins, by anaerobic bacteria in the intestine. Free p-cresol formed in this way is absorbed from the intestine and eliminated in the urine as conjugates.
WHO; Environ Health Criteria 168: Cresols (1995). Available from, as of December 22, 2014: https://www.inchem.org/pages/ehc.html
For more Absorption, Distribution and Excretion (Complete) data for p-CRESOL (9 total), please visit the HSDB record page.
p-Cresol fed to rabbits is excreted in the urine as the glucuronide (60%) and sulfate (15%) conjugates, some 10% is oxidized to p-hydroxybenzoic acid and a trace is hydroxylated to 3,4-dihydroxytoluene.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 147
p-Cresol yields p-cresyl-beta-d-glucuronide, p-cresyl sulfate, p-hydroxybenzyl alcohol, 4-methylcatechol, and p-methylanisole in rabbits.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-59
p-Cresol yields p-cresyl sulfate in man.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-59
p-Cresol yields p-cresyl sulfate and p-methylanisole in rats.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-59
For more Metabolism/Metabolites (Complete) data for p-CRESOL (18 total), please visit the HSDB record page.
4-Methylphenol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylic acid, 4-Hydroxybenzyl alcohol, 4-Methyl-2,5-cyclohexadien-1-one, and 4-methyl-hydroquinone.
4-Methylphenol is a known human metabolite of toluene.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560