


1. Cyclo((4r)-4-(2-aminoethylcarbamoyloxy)-l-prolyl-l-phenylglycyl-d-tryptophyl-l-lysyl-4-o-benzyl-l-tyrosyl-l-phenylalanyl-)
2. Som 230
3. Som-230
4. Som230
1. Som 230
2. Som-230
3. Som230
4. 396091-73-9
5. Pasireotide Free Base
6. Chebi:72312
7. Signifor Lar
8. Chembl3349607
9. 396091-73-9 (free Base)
10. 98h1t17066
11. Pasireotide Diaspartate
12. Cyclo((4r)-4-(2-aminoethylcarbamoyloxy)-l-prolyl-l-phenylglycyl-d-tryptophyl-l-lysyl-4-o-benzyl-l-tyrosyl-l- Phenylalanyl-)
13. Cyclo((4r)-4-(2-aminoethylcarbamoyloxy)-l-prolyl-l-phenylglycyl-d-tryptophyl-l-lysyl-4-o-benzyl-l-tyrosyl-l-phenylalanyl-)
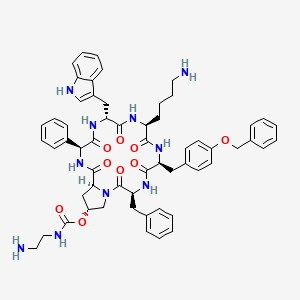
14. (3s,6r,9s,12s,15s,19r,20as)-6-((1h-indol-3-yl)methyl)-9-(4-aminobutyl)-15-benzyl-12-(4-(benzyloxy)benzyl)-1,4,7,10,13,16-hexaoxo-3-phenylicosahydropyrrolo[1,2-a][1,4,7,10,13,16]hexaazacyclooctadecin-19-yl (2-aminoethyl)carbamate
15. Cyclo[(2s)-2-phenylglycyl-d-tryptophyl-l-lysyl-o-benzyl-l-tyrosyl-l-phenylalanyl-(4r)-4-{[(2-aminoethyl)carbamoyl]oxy}-l-prolyl]
16. Pasireotide [inn]
17. Pasireotide [usan]
18. Pasireotide [usan:inn]
19. Pasireotida
20. Pasireotidum
21. Unii-98h1t17066
22. Pasireotide [mi]
23. Pasireotide [vandf]
24. Pasireotide [mart.]
25. Pasireotide [who-dd]
26. Gtpl2018
27. Schembl12462108
28. Som 320
29. Bdbm50474236
30. Db06663
31. Ncgc00378682-03
32. Hy-16381
33. Q3896970
34. [(3s,6s,9s,12r,15s,18s,20r)-9-(4-aminobutyl)-12-(1h-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-15-phenyl-6-[[4-(phenylmethoxy)phenyl]methyl]-3-(phenylmethyl)-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosan-20-yl] N-(2-aminoethyl)carbamate
35. [(3s,6s,9s,12r,15s,18s,20r)-9-(4-aminobutyl)-3-benzyl-12-(1h-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-15-phenyl-6-[(4-phenylmethoxyphenyl)methyl]-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosan-20-yl] N-(2-aminoethyl)carbamate
| Molecular Weight | 1047.2 g/mol |
|---|---|
| Molecular Formula | C58H66N10O9 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 18 |
| Exact Mass | 1046.50142372 g/mol |
| Monoisotopic Mass | 1046.50142372 g/mol |
| Topological Polar Surface Area | 281 Ų |
| Heavy Atom Count | 77 |
| Formal Charge | 0 |
| Complexity | 1940 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of Cushings disease, specifically for those patients whom pituitary surgery has not been curative or is not an option.
FDA Label
Signifor is indicated for the treatment of adult patients with Cushings disease for whom surgery is not an option or for whom surgery has failed.
Signifor is indicated for the treatment of adult patients with acromegaly for whom surgery is not an option or has not been curative and who are inadequately controlled on treatment with another somatostatin analogue.
Treatment of acromegaly and pituitary gigantism
Overproduction of pituitary ACTH, Pituitary dependant Cushing's disease, Pituitary dependant hyperadrenocorticism
Signifor is an analogue of somatostatin that promotes reduced levels of cortisol secretion in Cushing's disease patients.
Hormones
Chemical substances having a specific regulatory effect on the activity of a certain organ or organs. The term was originally applied to substances secreted by various ENDOCRINE GLANDS and transported in the bloodstream to the target organs. It is sometimes extended to include those substances that are not produced by the endocrine glands but that have similar effects. (See all compounds classified as Hormones.)
H01CB05
H - Systemic hormonal preparations, excl. sex hormones and insulins
H01 - Pituitary and hypothalamic hormones and analogues
H01C - Hypothalamic hormones
H01CB - Somatostatin and analogues
H01CB05 - Pasireotide
Absorption
The peak plasma concentration of pasireotide occurs in 0.25-0.5 hours. After administration of single and multiple doses, there is dose-proportionoal increases in Cmax and AUC.
Route of Elimination
Pasireotide is eliminated mostly by hepatic clearance (biliary excretion)(about 48%) with some minor renal clearance (about 7.63%).
Volume of Distribution
Pasireotide is widely distributed and has a volume of distribution of >100L.
Clearance
The clearance in healthy patient is ~7.6 L/h and in Cushings disease patients is ~3.8 L/h.
Metabolism is minimal.
The half-life is 12 hours.
Pasireotide activates a broad spectrum of somatostatin receptors, exhbiting a much higher binding affinity for somatostatin receptors 1, 3, and 5 than octreotide in vitro, as well as a comparable binding affinity for somatostatin receptor 2. The binding and activation of the somatostatin receptors causes inhibition of ACTH secretion and results in reduced cortisol secretion in Cushing's disease patients. Also this agent is more potent than somatostatin in inhibiting the release of human growth hormone (HGH), glucagon, and insulin.