


1. 2589 R.b.
2. Abactal
3. Dihydrate, Pefloxacin Mesylate
4. Mesylate Dihydrate, Pefloxacin
5. Mesylate, Pefloxacin
6. Peflacine
7. Pefloxacin
8. Pefloxacin Mesylate
9. Pefloxacin, Silver
10. Pefloxacine
11. R.b., 2589
12. Silver Pefloxacin
1. 149676-40-4
2. Peflacine
3. Peflox
4. Pefloxacin (mesylate Dihydrate)
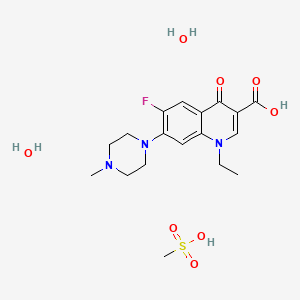
5. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic Acid
6. Cx28qc6fu0
7. Pefloxacin Mesilate Dihydrate
8. Pefloxacin Mesylate Dihydrate
9. Pefloxacinium Mesylate Dihydrate
10. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic Acid;methanesulfonic Acid;dihydrate
11. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Methanesulfonate Dihydrate
12. Eu 5306
13. Unii-cx28qc6fu0
14. Peflacin
15. Pefloxacin Mesylate Dihydrate [ep]
16. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Mesylate Dihydrate
17. Mfcd01685696
18. Rb 1589
19. Pefloxacinmesylatedihydrate
20. Hy-b0147b
21. 1589mrb
22. Hms3656n18
23. S4119
24. Akos015896973
25. Ccg-269406
26. Cs-1963
27. Ks-1095
28. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Compound With Methanesulfonic Acid (1:1) Dihydrate
29. Db-043005
30. Ft-0631178
31. Sw197608-4
32. A836889
33. Pefloxacin Mesylate Dihydrate [ep Impurity]
34. Pefloxacin Methanesulfonate Dihydrate [mi]
35. Pefloxacin Mesilate Dihydrate [ep Monograph]
36. Q27275871
37. Pefloxacin Mesilate Dihydrate, European Pharmacopoeia (ep) Reference Standard
38. 1-ethyl-6-fluoranyl-7-(4-methylpiperazin-1-yl)-4-oxidanylidene-quinoline-3-carboxylic Acid; Methanesulfonic Acid; Dihydrate
39. 1-ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid; Methanesulfonic Acid; Dihydrate
| Molecular Weight | 465.5 g/mol |
|---|---|
| Molecular Formula | C18H28FN3O8S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Exact Mass | 465.15811420 g/mol |
| Monoisotopic Mass | 465.15811420 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 638 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Cytochrome P-450 CYP1A2 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inhibitors.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)