


1. 2589 R.b.
2. Abactal
3. Dihydrate, Pefloxacin Mesylate
4. Mesylate Dihydrate, Pefloxacin
5. Mesylate, Pefloxacin
6. Peflacine
7. Pefloxacin Mesylate
8. Pefloxacin Mesylate Dihydrate
9. Pefloxacin, Silver
10. Pefloxacine
11. R.b., 2589
12. Silver Pefloxacin
1. 70458-92-3
2. Pefloxacine
3. Pefloxacino
4. Pefloxacinum
5. Pflx
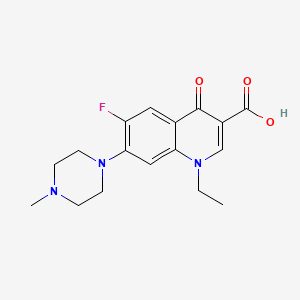
6. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
7. Pefloxacinium
8. Eu-5306
9. 1589 Rb
10. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic Acid
11. Eu 5306
12. Pefloxacinium Mesylate
13. Chebi:50199
14. 2h52z9f2q5
15. 3-quinolinecarboxylic Acid, 1,4-dihydro-1-ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-
16. 3-quinolinecarboxylic Acid,1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-
17. 1589-rb
18. Pefloxacin [inn-french]
19. Pefloxacinum [inn-latin]
20. Pefloxacino [inn-spanish]
21. Perfloxacin
22. Am-725
23. Labocton
24. Mls001049009
25. 1584rb
26. Smr000273037
27. Sr-01000356843
28. Pefloxacin (usan/inn)
29. Smr000387024
30. Einecs 274-611-8
31. Brn 0567618
32. Pefloxacin [usan:inn:ban]
33. Unii-2h52z9f2q5
34. Ccris 8210
35. Pefloxacin,(s)
36. 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-methyl-1-piperazinyl)-3-quinolinecarboxylic Acid
37. 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
38. Bas 00463355
39. Pefloxacin [mi]
40. Pefloxacin [inn]
41. Spectrum2_000034
42. Spectrum3_001971
43. Pefloxacin [usan]
44. 3-quinolinecarboxylic Acid, 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo
45. 3-quinolinecarboxylic Acid, 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-
46. Pefloxacin [who-dd]
47. Oprea1_063677
48. Oprea1_546341
49. Schembl34292
50. Bspbio_003571
51. Mls000713556
52. Mls006011797
53. Spbio_000127
54. Chembl267648
55. Cid_119525
56. Zinc1894
57. Dtxsid3048493
58. Bdbm57936
59. Kbio3_002945
60. Hms1613e05
61. Hms2090h09
62. Hms3713g04
63. Bcp20511
64. Hy-b0147
65. Bbl008795
66. Mfcd00865015
67. Stk169677
68. Akos000280245
69. Ccg-111464
70. Cs-1961
71. Db00487
72. Ncgc00177986-04
73. Vs-01991
74. Sbi-0206758.p001
75. Db-055417
76. Ft-0630798
77. Vu0243371-6
78. D02306
79. P-1198
80. Ab00600922-12
81. Ab00600922-14
82. Ab00600922_15
83. Ab00600922_16
84. 458p923
85. Pefloxacin, Antibiotic For Culture Media Use Only
86. Q2601859
87. Sr-01000356843-1
88. Sr-01000356843-6
89. Brd-k55034111-001-02-0
90. Brd-k55034111-066-02-3
91. Brd-k55034111-066-07-2
92. F5577-0091
93. 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-methylpiperazinyl)quinoline-3-carboxylic Acid
94. 1-ethyl-6-fluoro-1,4-dihydro-7-(4-methyl-1- Piperazinyl)-4-oxo-3-quinolinecarboxylic Acid
95. 1-ethyl-6-fluoro-4-keto-7-(4-methylpiperazino)quinoline-3-carboxylic Acid;mesylic Acid
96. 1-ethyl-6-fluoro-7-(4-methyl-piperazin-1-yl)-4-oxo-1,4-dihydro-quinoline-3-carbo
97. 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylicacid
98. 1-ethyl-6-fluoro-7-(4-methylpiperazin-4-ium-1-yl)-4-oxoquinoline-3-carboxylate
99. 1-ethyl-6-fluoranyl-7-(4-methylpiperazin-1-yl)-4-oxidanylidene-quinoline-3-carboxylic Acid;methanesulfonic Acid
100. 1-ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid;methanesulfonic Acid
| Molecular Weight | 333.36 g/mol |
|---|---|
| Molecular Formula | C17H20FN3O3 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 333.14886967 g/mol |
| Monoisotopic Mass | 333.14886967 g/mol |
| Topological Polar Surface Area | 64.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 545 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of uncomplicated gonococcal urethritis in males and for gram-negative-bacterial infections in the gastrointestinal system and the genitourinary tract.
Pefloxacin is a fluoroquinolone antibiotic. Flouroquinolones such as pefloxacin possess excellent activity against gram-negative aerobic bacteria such as E.coli and Neisseria gonorrhoea as well as gram-positive bacteria including S. pneumoniae and Staphylococcus aureus. They also posses effective activity against shigella, salmonella, campylobacter, gonococcal organisms, and multi drug resistant pseudomonas and enterobacter.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Cytochrome P-450 CYP1A2 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA03 - Pefloxacin
Absorption
Well absorbed by the oral route.
Hepatic. Primary metabolites are pefloxacin N-oxide and norfloxacin.
8.6 hours
The bactericidal action of pefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited.
