

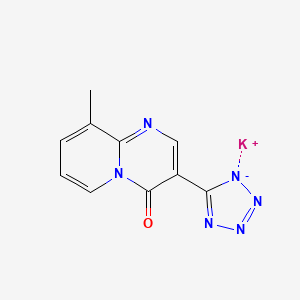

1. 9-methyl-3-(1h-tetrazol-5-yl)-4h-pyrido(1,2-a)pyrimidin-4-one
2. 9-tbx
3. Alamast
4. Bmy 26517
5. Bmy-26517
6. Pemirolast
7. Pemirolast Potassium Salt
1. 100299-08-9
2. Alamast
3. Pemirolast Potassium Salt
4. Pemilaston
5. Alegysal
6. Bmy 26517
7. Bmy-26517
8. Twt-8152
9. 497a17ouue
10. Tbx
11. 100299-08-9 (potassium)
12. 9-methyl-3-(1h-tetrazol-5-yl)-4h-pyrido(1,2-a)pyrimidin-4-one, Potassium Salt
13. Potassium 5-(9-methyl-4-oxo-4h-pyrido[1,2-a]pyrimidin-3-yl)tetrazol-1-ide
14. 4h-pyrido(1,2-a)pyrimidin-4-one, 9-methyl-3-(1h-tetrazol-5-yl)-, Potassium Salt
15. Dsstox_cid_26623
16. Dsstox_rid_81773
17. Dsstox_gsid_46623
18. 9-methyl-3-(1h-tetrazol-5-yl)-4h-pyrido(1,2-a)pyrimidin-4-one Potassium Salt
19. 9-methyl-3-(1h-tetrazol-5-yl)-4h-pyrido[1,2-a]pyrimidin-4-one Potassium Salt
20. Ccris 3562
21. Cas-100299-08-9
22. Pemirolast Potassium [usan:jan]
23. Ncgc00167434-01
24. Unii-497a17ouue
25. Pemilaston (tn)
26. Alegysal (tn)
27. Alamast (tn)
28. Permirolast Potassium
29. Pemirolast Potassium- Bio-x
30. Alamast, Pemirolast Potassium
31. Schembl865789
32. Chembl1201050
33. Dtxsid7046623
34. Hms3713g17
35. Pemirolast Potassium [jan]
36. Pemirolast Potassium [usan]
37. Bcp21138
38. Pemirolast Potassium (jp17/usan)
39. Pemirolast Potassium [vandf]
40. Tox21_112438
41. Bdbm50248065
42. De-068
43. Pemirolast (bmy 26517) Potassium
44. Pemirolast Potassium [mart.]
45. Permirolast Potassium [vandf]
46. S4008
47. Pemirolast Potassium [who-dd]
48. Akos015901748
49. Pemirolast Potassium Salt [mi]
50. Tox21_112438_1
51. Ac-8303
52. Ccg-220358
53. Hs-0020
54. Pemirolast Potassium, >=98% (hplc)
55. Sb18793
56. Ncgc00167434-02
57. Ac-22387
58. Bp164244
59. Pemirolast Potassium [orange Book]
60. Ft-0631005
61. P1995
62. P2547
63. D01088
64. T71519
65. 299p089
66. J-000087
67. Q27259246
68. 9-methyl-3-(2h-tetrazol-5-yl)-4h-pyrido[1,2-a]pyrimidin-4-one Potassium Salt;alamast
69. Potassium;9-methyl-3-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)pyrido[1,2-a]pyrimidin-4-one
| Molecular Weight | 266.30 g/mol |
|---|---|
| Molecular Formula | C10H7KN6O |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 266.03184035 g/mol |
| Monoisotopic Mass | 266.03184035 g/mol |
| Topological Polar Surface Area | 72.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)