
1. Dilcoran
2. Erinit
3. Nirason
4. Nitrodex
5. Pentalog
6. Peritrate
7. Tetranitrate, Pentaerythritol
8. Tetrate
1. Petn
2. Nitropentaerythrite
3. Pentaerithrityl Tetranitrate
4. Penthrite
5. Nitropentaerythritol
6. 78-11-5
7. Nitropenta
8. Peritrate
9. Pentanitrine
10. Pentanitrol
11. Vasodiatol
12. Angicap
13. Baritrate
14. Corpent
15. Pentaerythrityl Tetranitrate
16. Penthrit
17. Perityl
18. Quintrate
19. Antora
20. Erinit
21. Peridex-la
22. Pentritol Tempules
23. Deltrate-20
24. Neopentanetetrayl Nitrate
25. Arcotrate
26. Cardiacap
27. Dipentrate
28. Duotrate
29. Hasethrol
30. Kaytrate
31. Lowetrate
32. Metranil
33. Mycardol
34. Niperyth
35. Nitrinal
36. Nitrolong
37. Nitropent
38. Nitropenton
39. Nitrotalans
40. Pentafin
41. Pentitrate
42. Pentrate
43. Pentrite
44. Pentritol
45. Pentryate
46. Pergitral
47. Prevangor
48. Rythritol
49. Subicard
50. Tanipent
51. Tetrasule
52. Angitet
53. Lentrat
54. Niperyt
55. Pencard
56. Terpate
57. Extex
58. Chot
59. Pent
60. Nitro-riletten
61. Neo-corovas
62. Pen-tetra
63. Nitropenta 7w
64. Tetranitropentaerythrite
65. Tranite D-lay
66. Pentestan-80
67. Dilcoran-80
68. Martrate-45
69. Myotrate 10
70. Tentrate-20
71. Pentral 80
72. C 2 (explosive)
73. Pentetrate Unicelles
74. Pentaerythrite Tetranitrate
75. Tetranitropentaerythritol
76. Pentaerythritylium Tetranitricum
77. 1,3-dinitrato-2,2-bis(nitratomethyl)propane
78. 2,2-bis(hydroxymethyl)-1,3-propanediol Tetranitrate
79. Pentalong
80. [3-nitrooxy-2,2-bis(nitrooxymethyl)propyl] Nitrate
81. Pentaerithrityli Tetranitras
82. Xtx 8003
83. Tetranitrato De Pentaeritritilo
84. Tetranitrate De Pentaerithrityle
85. 1,3-propanediol, 2,2-bis[(nitrooxy)methyl]-, Dinitrate (ester)
86. 1,3-propanediol, 2,2-bis((nitrooxy)methyl)-, Dinitrate (ester)
87. 2,2-bisdihydroxymethyl-1,3-propanediol Tetranitrate
88. 10l39trg1z
89. Pentaerythritol Tetranitrate With 80% D-lactose Monohydrate
90. Chebi:25879
91. Pentaerithrityl Tetranitrate [inn]
92. Ncgc00159388-02
93. Pentanitrolum
94. Erynitum
95. Mikardol
96. Nitrinol
97. Pentarit
98. Pentriol
99. Vasitol
100. 2,2-bis((nitrooxy)methyl)-1,3-propanediol Dinitrate (ester)
101. Dsstox_cid_1109
102. Dsstox_rid_77024
103. Pentryate 80
104. Dsstox_gsid_23430
105. Deltrate 20
106. Dilcoran 80
107. Martrate 45
108. Vaso-80 Unicelies
109. 2,2-bis((nitrooxy)methyl)-1,3-propanediol Dinitrate
110. 3-(nitrooxy)-2,2-bis[(nitrooxy)methyl]propyl Nitrate
111. El Petn
112. Pentaerythritol Nitrate
113. 2,2-bis((nitrooxy)methyl)-1,3-propanediol Dinitrate Ester
114. Lx 16 (explosive)
115. Vaso-80
116. 1,3-propanediol, 2,2-bis((nitrooxy)methyl)-, 1,3-dinitrate
117. 2,2-bis[(nitrooxy)methyl]-1,3-propanediol Dinitrate (ester)
118. Pentaerithrityl Tetranitrate (inn)
119. Pentaerythritol Tetranitrate (jan)
120. Cas-78-11-5
121. Pentaerythritol, Tetranitrate
122. Pentaerythritol Tetranitrate [jan]
123. Sdm No. 23
124. Ten [vasodilator]
125. Ccris 2387
126. Pbxn 301
127. Tetranitrate De Pentaerythrityle
128. Hsdb 6313
129. Lx 13
130. Nci-c55743
131. Pentaeritrile Tetranitrato [dcit]
132. Pentaeritrile Tetranitrato
133. Petn, Nf
134. Pentaerythritoltetranitrate
135. Einecs 201-084-3
136. Pentaerythritol Tetranitrate, Diluted
137. Na0150
138. Un0411
139. Lx 02-1
140. Lx 08-0
141. Brn 1716886
142. Unii-10l39trg1z
143. Pentafilin
144. Pentaerithrityli Tetranitras [inn-latin]
145. Pentrinat
146. Tetranitrate De Pentaerithrityle [inn-french]
147. Tetranitrato De Pentaeritritilo [inn-spanish]
148. Vaso-80 Unicelles
149. Miltrate (salt/mix)
150. Pentaerythritol Tetranitrate [usp:jan]
151. Pentritol Tempules (tn)
152. Pentaerythrite Tetranitrate (dry) [forbidden]
153. Pentaerithritol Tetranitrate
154. Ec 201-084-3
155. Schembl37177
156. 4-01-00-02816 (beilstein Handbook Reference)
157. Sdm No. 23 (salt/mix)
158. Sdm No. 35 (salt/mix)
159. Chembl466659
160. P.e.t.n.
161. Dtxsid2023430
162. Pentaerythrite Tetranitrate (dry)
163. Pentaerythrite Tetranitrate, With Not < 7% Water, By Mass
164. Zinc8101167
165. Tox21_111624
166. Tox21_301747
167. C0051
168. Db06154
169. Ncgc00159388-03
170. Ncgc00159388-04
171. Ncgc00159388-05
172. Ncgc00255290-01
173. Nitropenta 10 Microg/ml In Acetonitrile
174. Pentaerythritol Tetranitrate [mi]
175. Pentaerythrite Tetranitrate, Wetted With Not < 25% Water, By Mass Or Desensitized With Not < 15% Phlegmatizer, By Mass
176. Pentaerythritol Tetranitrate [hsdb]
177. Pentaerithrityl Tetranitrate [mart.]
178. Pentaerythritol Tetranitrate [vandf]
179. Pentaerithrityl Tetranitrate [who-dd]
180. D01721
181. Q189334
182. 2,2-bis(hydroxy-methyl)-1,3-propanediol Tetranitrate
183. 1,3-propanediol, 2,2-bis[(nitrooxy)methyl]-, Dinitrate
184. 1,3-propanediol, 2,2-bis[(nitrooxy)methyl]-, Dinitrate (ester)
185. Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, Wetted With Not <25% Water, By Mass Or Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, Desensitized With Not <15% Phlegmatizer By Mass
186. Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, Wetted With Not <25% Water, By Mass Or Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, Desensitized With Not <15% Phlegmatizer By Mass [na0150] [explosive 1.1a]
187. Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, With Not <7% Wax By Mass
188. Pentaerythrite Tetranitrate Or Pentaerythritol Tetranitrate Or Petn, With Not <7% Wax By Mass [un0411] [explosive 1.1d]
189. Pentaerythritol Tetranitrate Solution, 10 Mg/ml In Acetonitrile, Ampule Of 5 Ml, Certified Reference Material
190. Pentaerythritol Tetranitrate Solution, 1000 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
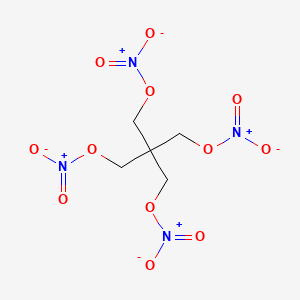
| Molecular Weight | 316.14 g/mol |
|---|---|
| Molecular Formula | C5H8N4O12 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 316.01387170 g/mol |
| Monoisotopic Mass | 316.01387170 g/mol |
| Topological Polar Surface Area | 220 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 311 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Vasodilator (coronary)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1228
FDA has classified the oral forms of pentaerythritol tetranitrate as possibly effective in the prophylaxis of angina pectoris, but not in the treatment of acute attacks. This classification requires the submission of adequate and well controlled studies in order to provide substantial evidence of effectiveness.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2025 (1992)
Drug preparations intended for human use containing "coronary vasodilators". New drug applications have been approved for products containing pentaerythritol tetranitrate.
21 CFR 250.102(a)(2) (USFDA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. Available from, as of January 4, 2010: https://www.ecfr.gov
For more Therapeutic Uses (Complete) data for Pentaerythritol tetranitrate (14 total), please visit the HSDB record page.
Transient headache and nausea may accompany its use ... Medical authorities state that the sustained release forms are poorly effective. It is not absorbed sublingually. Since absorption by the oral route is erratic, efficacy is unpredictable. /Pentaerythritol tetranitrate, diluted/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 794
... Elderly patients may be more sensitive to the hypotensive effects. In addition, elderly patients are more likely to have age related renal function impairment, which may require caution in patients receiving nitrates. /Nitrates (systemic)/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2026 (1992)
Nitrate therapy should be discontinued if blurred vision or dry mouth continues or is severe. /Nitrates (systemic)/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2027 (1992)
/Pentaerythritol/ tetranitrate is much longer acting than nitroglycerin but is slower in onset of action. Hence it is used in the prophylaxis of attacks of angina pectoris but not in the management of the acute attack. It is no better than a placebo as a routine chronic prophylactic in angina pectoris; tolerance develops with chronic use. /Pentaerythritol tetranitrate, diluted/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 794
In a randomized, double-blind, four-way crossover study, 24 healthy volunteers received 240 mg/d pentaerithritol tetranitrate (PETN), 150 mg/d PETN, 60 mg/d isosorbide mononitrate slow release (ISMN) or placebo in each study period for two days. Headache and disability to work were self-rated six times per day; individual measurements were combined to total scores. ISMN caused headaches more frequently (in approx. 90% of volunteers) and more severe (average total score 15.2) and a greater disability (average total score 6.0) than the high or low PETN-dosage (both in approx. 50%, headache score 4.9 or 6.4, disability score 1.1 or 2.1, resp.) and placebo (in approx. 10%, headache 0.8, disability 0), all these differences were statistically significant (p < 0.01, Wilcoxon). The high PETN-dosage showed a non-significant trend to produce fewer systemic side effects than the low PETN-dosage (not vice versa). With ISMN six volunteers prematurely terminated the study period and one volunteer who was replaced withdrew from the entire study due to side effects; all volunteers completed the study periods with the other medications.
PMID:9689421 Pfaffenrath V et al; Arzneimittelforschung 48 (6): 646-50 (1998).
Used for the treatment of angina pectoris.
Organic nitrate which causes systemic vasodilation, decreasing cardiovascular preload. Nitrate enters vascular smooth muscle and converted to nitric oxide (NO) which acts as a cellular messenger, leading to activation of cyclic GMP and, therefore, vasodilation. The nitrovasodilator group of drugs relaxes most smooth muscles in the body, including those in the walls of arteries and veins, and selectively dilate large coronary vessels. Lower doses of nitrates increase coronary blood flow without significantly affecting systemic arterial pressure. Higher doses, especially if repeated frequently, decrease systolic and diastolic blood pressure as well as cardiac output, which can result in a headache, weakness, dizziness, and the activation of compensatory sympathetic reflexes, including tachycardia and peripheral arteriolar vasoconstriction. Smooth muscles in the bronchi, biliary tract, gastrointestinal tract, ureters, and uterus also can be relaxed by nitrovasodilators. PETN seems to be unique among the long-acting nitrovasodilators in that patients do not demonstrate tolerance to treatment, which results in sustained vasodilation in humans with continuous PETN treatment. Important to note is that this drug is devoid of induction of oxidative stress and related side-effects such as endothelial dysfunction or tolerance to nitrates. Some of these effects are related to special pharmacokinetics of PETN, but upon chronic administration, PETN also induces antioxidative pathways at the genomic level, resulting in increased expression of heme _oxygenase-1 (HO-1)_ _and ferritin_, both possessing highly protective properties. There is good experimental evidence that at least part of the beneficial profile of long-term treatment with this drug is based on the activation of the _heme oxygenase-1/ferritin_ system.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DA - Organic nitrates
C01DA05 - Pentaerithrityl tetranitrate
Route of Elimination
Mainly the urine.
Volume of Distribution
The steady-state volume of distribution was 4.2 +/- 1.1 L/kg (n = 6) in rats given this drug by the intra-arterial route.
Clearance
In a pharmacokinetic study of rats after intra-arterial administration of this drug, the clearance was measured to be 0.61 +/- 0.16 L/min/kg.
During the first 4 hr after ... /pentaerythritol tetranitrate/ admin /in humans/ ... there were significant blood levels of pentaerythritol dinitrate.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 173
PETN is absorbed slowly from the gastrointestinal tract and lung but not appreciably through the skin.
Doepker CL; Patty's Toxicology CD-ROM (2005). NY, NY: John Wiley & Sons; Aliphatic Nitro, Nitrate, And Nitrite Compounds. Online Posting Date: April 16, 2001
The systemic absorption of meprobamate, diphenhydramine and pentaerythritol tetranitrate (PETN) has been demonstrated following oral administration of a formulation containing all three drug substances to human volunteers. A study undertaken in dogs has also been made of the pharmacokinetics of the major nitrated metabolite of PETN when the parent drug is administered with and without meprobamate and diphenhydramine. Pentaerythritol mononitrate shows a six-fold increase in both peak plasma concentrations and area under the 0-12 hour plasma concentration-time curve when PETN is co-administered with a combination of meprobamate, diphenhydramine and nicotinic acid. No such increase is apparent when either meprobamate or diphenhydramine is excluded from the dose. Further increases in pentaerythritol mononitrate plasma levels and AUC 0-12 hr are observed when all of the drugs are administered as the formulated coated tablet (VisanoCor).
PMID:7201836 Gilbert JD et al; Arzneimittelforschung 32 (5): 571-4 (1982).
Following oral admin of (14)carbon labeled pentaerythritol tetranitrate in one study in fasting subjects, 60% and 50% of a 20 mg and 40 mg dose, respectively, were absorbed. Following oral admin of the drug in a tablet form, the onset of hemodynamic effects is about 20-60 min and the duration of action is 4-5 hr.
American Hospital Formulary Service-Drug Information 85. Bethesda, MD: American Society Hospital Pharmacists, 1985. (Plus supplements A & B, 1985)., p. 694
For more Absorption, Distribution and Excretion (Complete) data for Pentaerythritol tetranitrate (10 total), please visit the HSDB record page.
Extensively metabolized in the liver. Metabolites: pentaerythritol trinitrate, pentaerythritol dinitrate, pentaerythritol mononitrate, & pentaerythritol (inactive)
Rapid de-esterfication of pentaerythritol tetranitrate after po dosing to humans resulted in measurable serum levels of pentaerythritol, pentaerythritol pentaerythritol mononitrate, and small amt of the dinitrate, but no unchanged drug. The kinetics of urinary excretion of pentaerythritol tetranitrate were first-order and dose-dependent. The ratio of mononitrate to pentaerythritol excreted in urine was 3:1 from a 40 mg dose and 1:1 from a 20 mg dose, suggesting a limited capacity for conversion of pentaerythritol mononitrate into pentaerythritol.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 446
The main metabolites were found to be pentaerythritol and the mononitrate ester; unchanged drug was recovered from feces only, suggesting a partial hydrolysis mediated by the intestinal microflora to be necessary prior to absorption /in man/.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 141
Pentaerythritol tetranitrate is metabolized primarily to ... pentaerythritol trinitrate (pertrinitrol) ... . The trinitrate, dinitrate, and mononitrate metabolites may undergo glucuronide conjugation. The plasma half-life of pentaerythritol trinitrate, which is therapeutically active, is about 10 min. The other metabolites are inactive.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1022
The elimination half-life in plasma from male volunteers given an oral 100-mg dose of the tetranitrate was reported to be 4-5 hours.
The elimination half-life in plasma from male volunteers given an oral 100-mg dose of the tetranitrate was reported to be 4-5 hours for the dinitrate and 10-11 hours for the mononitrate of pentaerythritol.
U.S. Department of Labor/Occupational Safety and Health Administration's Index of Sampling and Analytical Methods. Pentaerythritol tetranitrate (78-11-5). Available from, as of December 30, 2009: https://www.osha.gov/dts/chemicalsampling/toc/toc_chemsamp.html
Pentaerythritol tetranitrate is the lipid soluble polyol ester of nitric acid belonging to the family of _nitro-vasodilators_. Pentaerythritol tetranitrate releases free nitric oxide (NO) after the denitration reaction, which triggers NO-dependent signaling transduction involving soluble _guanylate cyclase (sGC_). Nitric oxide binds reversibly to the ferrous-heme center of sGC, causing conformational change and activating the enzyme. This enzyme activation results in increased cellular concentrations of _cyclic guanosine monophosphate _(cGMP) within the vascular smooth muscle, resulting in vasodilation mediated by cGMP-dependent protein kinases. Additionally, this agent causes dose-dependent arterial and venous bed.
The nitrates reduce myocardial oxygen requirements through their effects on the systemic circulation. Their systemic actions include (1) a reduction in venous tone, which leads to pooling of blood in peripheral veins, decr venous return, and reduced ventricular volume and myocardial tension (preload); and (2) a decr in peripheral vascular resistance, which reduces arterial blood pressure and ventricular outflow resistance (afterload). /Nitrates/
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 527
Antianginal or cardiac load reducing agent: /Mechanism of action/ not specifically known but thought to cause a reduction of myocardial oxygen demand. This is attributed to a reduction in left ventricular preload and afterload because of venous (predominantly) and arterial dilation with a more efficient redistribution of blood flow within the myocardium. Antihypertensive: Peripheral vasodilation. /Nitrates (systemic)/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2025 (1992)
The basic mechanisms responsible for the vasodilatory and anti-ischemic action of organic nitrates involve bioactivation of, and nitric oxide (NO) release from, these compounds which have therefore been termed NO donors. The organic nitrate pentaerythritol tetranitrate (PETN) is known to possess antioxidant properties that are thought to be the underlying cause for its specific pharmacological profile. In contrast to other long-acting nitrates, PETN induces tolerance- free vasodilation in humans and was reported to prevent endothelial dysfunction as well as atherogenesis in cholesterol- fed rabbits. ... The active PETN metabolite PETriN stimulates protein expression of the antioxidant defense protein heme oxygenase-1 (HO-1). Additionally, PETriN enhanced the enzymatic activity of HO-1 measured as formation of the HO-1 metabolites bilirubin and carbon monoxide in lysates from endothelial cells. HO-1 induction subsequently led to a marked increase in protein expression of a second antioxidant protein, ferritin, via the HO-1-dependent release of free iron from endogenous heme sources. Pretreatment of endothelial cells with PETriN was followed by increased cellular resistance to oxidant injury mediated by hydrogen peroxide. Endothelial protection by PETriN was mimicked by exogenous bilirubin which led to an almost complete reversal of hydrogen peroxide-induced toxicity. Increased HO-1 and ferritin expression as well as endothelial protection occurred at micromolar concentrations of PETriN which are well within the range of plasma or tissue levels that can be expected during oral therapy. The capacity to protect the endothelium in vitro may translate into and explain the previously observed antiatherogenic actions of PETN in vivo. ... Moreover, in earlier investigations aimed at assessing the antiatherogenic potential of nitrates, PETN but not isosorbide nitrates prevented plaque formation and endothelial dysfunction in animal models of atherosclerosis. Thus, the ability to activate HO-1 induction and associated antioxidant pathways apparently distinguishes PETN from other long-acting nitrates and may explain their different patterns of action in vivo.
PMID:14968347 Grosser N, Schroder H; Herz 29 (1): 116-22 (2004). Available fron, as of December 31, 2009:
Nitrates vary in their potential to induce the development of tolerance. During long-lasting nitrate therapy, except pentaerythritol tetranitrate (PETN), one can observe the development of reactive oxygen species (ROS) inside the muscular cell of a vessel wall, and these bind with nitric oxide (NO). This leads to decreased NO activity, thus, nitrate tolerance. PETN has no tendency to form ROS, and therefore during long-term PETN therapy, there is probably no tolerance or cross-tolerance, as during treatment with other nitrates.
PMID:19442078 Kosmicki MA; Curr Clin Pharmacol 4 (2):132-41 (2009).