
1. Diabutal
2. Etaminal
3. Ethaminal
4. Mebubarbital
5. Mebumal
6. Monosodium Salt Pentobarbital
7. Nembutal
8. Pentobarbital Sodium
9. Pentobarbital, Monosodium Salt
10. Pentobarbitone
11. Sagatal
1. Pentobarbitone
2. Nembutal
3. Mebubarbital
4. Mebumal
5. Ethaminal
6. Pentobarbituric Acid
7. Neodorm
8. 76-74-4
9. Dorsital
10. Nebralin
11. Rivadorm
12. Pentobarbiturate
13. Pentabarbitone
14. Neodorm (new)
15. Pentabarbital
16. Pentobarbitalum
17. 5-ethyl-5-(sec-pentyl)barbituric Acid
18. 5-ethyl-5-(1-methylbutyl)barbituric Acid
19. 5-ethyl-5-(1-methylbutyl)malonylurea
20. 5-ethyl-5-(1-methylbutyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
21. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylbutyl)-
22. Nsc 28708
23. Sodium Pentobarbital
24. Pentobarbital Cii
25. Pentobarbital Calcium
26. Barbituric Acid, 5-ethyl-5-(1-methylbutyl)-
27. Nsc-28708
28. 5-ethyl-5-(pentan-2-yl)pyrimidine-2,4,6(1h,3h,5h)-trione
29. Chembl448
30. 5-ethyl-5-pentan-2-yl-1,3-diazinane-2,4,6-trione
31. Chebi:7983
32. Aethaminalum
33. I4744080ir
34. Nembutal (van)
35. Neodorm (van)
36. Ncgc00096074-01
37. Pentobarbitale
38. Pentobarbital (van)
39. Pentobarbitone (van)
40. Pentobarbitale [dcit]
41. 5-ethyl-5-(1-methyl-butyl)-pyrimidine-2,4,6-trione
42. Pentobarbitalum [inn-latin]
43. 5-ethyl-5-(pentan-2-yl)-1,3-diazinane-2,4,6-trione
44. Phetobarbitone
45. 5-ethyl-2-hydroxy-5-(1-methylbutyl)pyrimidine-4,6(1h,5h)-dione
46. Nembutal (tn)
47. Ethyl-propylmethylcarbinylbarbituric Acid
48. Ccris 7089
49. Pentobarbital [inn]
50. Hsdb 3151
51. Einecs 200-983-8
52. Pentobarbital (usp/inn)
53. Brn 0087067
54. Barbituric Acid, 5-ethyl-5-(2-pentyl)-
55. Pentobarbital [usp:inn:ban]
56. Sr-01000317092
57. Unii-i4744080ir
58. (+-)-5-ethyl-5-(1-methylbutyl)barbituric Acid
59. (rs)-pentobarbital
60. Continal (salt/mix)
61. (plusmn)-pentobarbital
62. Sedalixir (salt/mix)
63. Spectrum_001783
64. 57-33-0
65. Spectrum2_001991
66. Spectrum3_001783
67. Spectrum4_000574
68. Spectrum5_001705
69. Dsstox_cid_3435
70. Pentobarbital [mi]
71. Ec 200-983-8
72. (.+/-.))-pentobarbital
73. Dsstox_rid_77026
74. Pentobarbital [hsdb]
75. Dsstox_gsid_23435
76. Oprea1_143902
77. Oprea1_775730
78. Schembl24966
79. Bspbio_003305
80. Kbiogr_001008
81. Kbioss_002267
82. Pentobarbital [vandf]
83. 5-24-09-00168 (beilstein Handbook Reference)
84. Divk1c_000992
85. Pentobarbital [mart.]
86. Spectrum1900006
87. Spbio_002201
88. Pentobarbital [who-dd]
89. Gtpl5480
90. Dtxsid7023435
91. Schembl11114711
92. Component Of Emesert (salt/mix)
93. Component Of Synirin (salt/mix)
94. Hms503g05
95. Kbio1_000992
96. Kbio2_002266
97. Kbio2_004834
98. Kbio2_007402
99. Kbio3_002807
100. Ninds_000992
101. Hms2094e21
102. Hms3713b10
103. Pentobarbital [green Book]
104. Pharmakon1600-01900006
105. Pentobarbital [orange Book]
106. Pentobarbital Cii [usp-rs]
107. Nsc28708
108. Pentobarbital [ep Monograph]
109. Tox21_111555
110. Bdbm50055935
111. Ccg-39476
112. Nsc760434
113. Pentobarbital [usp Monograph]
114. Stl367899
115. Pentobarbital 0.1 Mg/ml In Methanol
116. Pentobarbital 1.0 Mg/ml In Methanol
117. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylbutyl)-, (+-)-
118. Akos000277861
119. Wln: T6vmvmv Fhj Fy3&1 F2
120. Ccg-220564
121. Db00312
122. Nsc-760434
123. Cas-76-74-4
124. Idi1_000992
125. Ncgc00096074-02
126. Ncgc00344563-01
127. Sbi-0052713.p002
128. Db-056119
129. C07422
130. D00499
131. 057p561
132. Q409632
133. Sr-05000001789
134. Thiopental Sodium Impurity B [ep Impurity]
135. Sr-01000317092-2
136. Sr-05000001789-1
137. Brd-a44448661-001-01-4
138. (+/-)-5-ethyl-5-(1-methylbutyl)barbituric Acid
139. 2,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylbutyl)-
140. 5-ethyl-5-(1-methylbutyl)-2,6(1h,3h,5h)-pyrimidinetrione
141. Pentobarbital, European Pharmacopoeia (ep) Reference Standard
142. Pentobarbital, United States Pharmacopeia (usp) Reference Standard
143. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylbutyl)-, (+/-)-
144. Pentobarbital Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 226.27 g/mol |
|---|---|
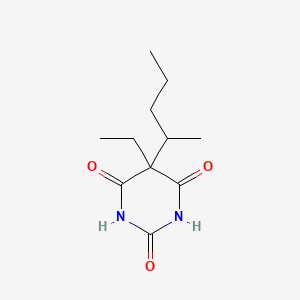
| Molecular Formula | C11H18N2O3 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 226.13174244 g/mol |
| Monoisotopic Mass | 226.13174244 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 305 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Nembutal sodium |
| Drug Label | The barbiturates are nonselective central nervous system depressants which are primarily used as sedative hypnotics and also anticonvulsants in subhypnotic doses. The barbiturates and their sodium salts are subject to control under the Federal Contro... |
| Active Ingredient | Pentobarbital sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Oak Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Nembutal sodium |
| Drug Label | The barbiturates are nonselective central nervous system depressants which are primarily used as sedative hypnotics and also anticonvulsants in subhypnotic doses. The barbiturates and their sodium salts are subject to control under the Federal Contro... |
| Active Ingredient | Pentobarbital sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Oak Pharms |
Adjuvants, Anesthesia; GABA Modulators; Sedatives, Barbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Pentobarbital sodium is indicated for use as a/ sedative. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
/Pentobarbital sodium is indicated for use as a/ hypnotic, for the short-term treatment of insomnia, since they appear to lose their effectiveness for sleep induction and sleep maintenance after 2 weeks. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
/Pentobarbital sodium is indicated for use as a/ preanesthetic. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (Pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
For more Therapeutic Uses (Complete) data for Pentobarbital (10 total), please visit the HSDB record page.
Barbiturates may be habit forming. Tolerance, psychological and physical dependence may occur with continued use. Patients who have psychological dependence on barbiturates may increase the dosage or decrease the dosage interval without consulting a physician and may subsequently develop a physical dependence on barbiturates. To minimize the possibility of overdosage or the development of dependence, the prescribing and dispensing of sedative-hypnotic barbiturates should be limited to the amount required for the interval until the next appointment. Abrupt cessation after prolonged use in the dependent person may result in withdrawal symptoms, including delirium, convulsions, and possibly death. Barbiturates should be withdrawn gradually from any patient known to be taking excessive dosage over long periods of time.
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
Nembutal Sodium capsules contain the dye tartrazine (FD&C yellow No. 5), which may cause allergic reactions including bronchial asthma in susceptible individuals. Although the incidence of tartrazine sensitivity is low, it frequently occurs in patients who are sensitive to aspirin.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
IV administered pentobarbital sodium may cause respiratory depression, apnea, laryngospasm, bronchospasm, or hypotension, particularly if the drug is administered too rapidly. When administered IV, the drug must be administered slowly, and personnel and equipment should be readily available for administration of artificial respiration. Too rapid administration may cause respiratory depression, apnea, laryngospasm, or vasodilation with fall in blood pressure.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
Barbiturates should be administered with caution, if at all, to patients who are mentally depressed, have suicidal tendencies, or a history of drug abuse.
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
For more Drug Warnings (Complete) data for Pentobarbital (34 total), please visit the HSDB record page.
The toxic dose of barbiturates varies considerably but, in general, a severe reaction is likely to occur when the amount ingested is more than 10 times the usual oral hypnotic dose. Potentially lethal blood concentrations are those in excess of 80 ug/mL for phenobarbital, 50 ug/mL for amobarbital or butabarbital, and approximately 30 ug/mL for secobarbital or pentobarbital; however, some patients have survived much higher blood concentrations. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2578
For the short-term treatment of insomnia.
Pentobarbital, a barbiturate, is used for the treatment of short term insomnia. It belongs to a group of medicines called central nervous system (CNS) depressants that induce drowsiness and relieve tension or nervousness. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA01 - Pentobarbital
Absorption
Barbiturates are absorbed in varying degrees following oral, rectal, or parenteral administration.
Route of Elimination
Barbiturates are metabolized primarily by the hepatic microsomal enzyme system, and the metabolic products are excreted in the urine, and less commonly, in the feces. Approximately 25 to 50 percent of a dose of aprobarbital or phenobarbital is eliminated unchanged in the urine, whereas the amount of other barbiturates excreted unchanged in the urine is negligible.
Nearly all of an oral or rectal dose of pentobarbital is absorbed from the GI tract. Following oral administration of pentobarbital, peak plasma concentrations are usually reached in 30-60 minutes.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
When pentobarbital is administered orally or rectally, the onset of action occurs within 15-60 minutes. The onset of action is within 1 minute following iv administration and within 10-25 minutes following im administration. Like secobarbital, pentobarbital probably has a duration of hypnotic effect of 1-4 hours following oral or rectal administration and about 15 minutes following iv administration.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
Plasma pentobarbital concentrations of 1-5 ug/mL generally produce sedation, and plasma concentrations of 5-15 ug/mL produce sleep in most patients; however, plasma concentrations of greater than 10 ug/mL may produce deep coma, and those in excess of 30 mcg/mL are potentially lethal.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
Approximately 35-45% of pentobarbital is bound to plasma proteins.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
For more Absorption, Distribution and Excretion (Complete) data for Pentobarbital (15 total), please visit the HSDB record page.
by hepatic microsomal enzyme system
Pentobarbital is metabolized by the liver chiefly by penultimate oxidation of the 1-methylbutyl substituent to a secondary alcohol, 5-ethyl-5-(3'-hydroxy-1'-methylbutyl) barbituric acid (hydroxypentobarbital) which is an inactive metabolite. Approximately 40-50% of an oral hypnotic dose of pentobarbital is excreted in urine as hydroxypentobarbital. The 1-methylbutyl substituent of pentobarbital can also be oxidized to form pentobarbital carboxylic acid, and small quantities of this metabolite have been found in urine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
The liver biotransforms most ... short acting barbiturates (90%-99%). Short-acting compounds /eg, pentobarbital & secobarbital/ are oxidized to more polar & inactive compounds (alcohols, ketones, phenol, or carboxylic acid). /Barbiturates/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 575
Pentobarbital contains an asymmetric carbon atom in butyl side chain. Main route of metabolism is (omega-1)-oxidation to yield 5-ethyl-5-(3'-hydroxy-1'-methylbutyl)barbituric acid. This process creates a new center of asymmetry, thus giving rise to 4 possible diastereoisomeric metabolites.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 257
Metabolized by liver by ... oxidation of 1-methylbutyl substituent to ... 5-ethyl-5-(3-hydroxy-1-methylbutyl)barbituric acid (hydroxypentobarbital) /and/ pentobarbital carboxylic acid ... Glucuronide conjugates of alcohols /and further unidentified oxidation products also found in urine of /humans/.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1975
For more Metabolism/Metabolites (Complete) data for Pentobarbital (6 total), please visit the HSDB record page.
5 to 50 hours (dose dependent)
Plasma concentrations of pentobarbital decline in a biphasic manner with a half-life of about 4 hours for the first phase and 35-50 hours for the second phase.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2583
The plasma half-life for pentobarbital in adults is 15 to 50 hours and appears to be dose dependent.
US Natl Inst Health; DailyMed. Current Medication Information for Nembutal sodium (pentobarbital sodium) injection (November 2006). Available from, as of March 19, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2563
Following iv admin of pentobarbitone sodium 50 mg to 5 healthy subjects pentobarbitone was noted to have a distribution phase (alpha-phase) of about 4 hr, & elimination occurred with a harmonic mean beta-phase half-life of about 50 hr. This suggested that for pentobarbitone the body has a central plasma compartment & one or more extravascular compartments. ... /In another study/ findings of 7 healthy subjects /revealed/ an average beta-phase half-life of only 22.3 hr following iv admin of 100 mg pentobarbitone sodium. After oral admin the half-life was about the same. A more detailed knowledge of pentobarbitone pharmacokinetics was needed to explain the deviation ... /between these 2 different studies/. /Pentobarbitone sodium/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 810
Pentobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged. All of these effects are associated with marked decreases in GABA-sensitive neuronal calcium conductance (gCa). The net result of barbiturate action is acute potentiation of inhibitory GABAergic tone. Barbiturates also act through potent (if less well characterized) and direct inhibition of excitatory AMPA-type glutamate receptors, resulting in a profound suppression of glutamatergic neurotransmission.
The exact mechanism(s) by which barbiturates exert their effect on the CNS, has not been fully elucidated. However, it is believed that such effects are related, at least partially, to the drugs' ability to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS, by altering inhibitory synaptic transmissions that are mediated by GABAA receptors. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticular formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Relatively low doses of the barbiturates depress the sensory cortex, decrease motor activity, and produce sedation and drowsiness. In some patients, however, drowsiness may be preceded by a period of transient elation, confusion, euphoria, or excitement, especially after subhypnotic doses of aprobarbital, pentobarbital, or secobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Larger doses distort judgment, cloud perception, suppress motor activity, and produce drowsiness and sleep. Still larger doses induce anesthesia. Barbiturate-induced sleep differs from physiologic sleep. Barbiturates reduce the rapid eye movement (REM) or dreaming stage of sleep. Stages III and IV sleep are also decreased. Although tolerance develops to the REM-suppressant effects during chronic administration, REM rebound occurs when the drugs are withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Mechanism of Action (Complete) data for Pentobarbital (22 total), please visit the HSDB record page.