

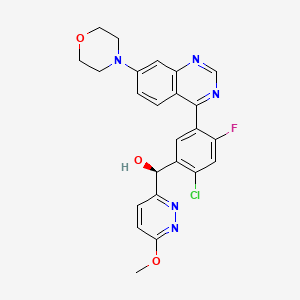

1. (s)-(2-chloro-4-fluoro-5-(7-morpholinoquinazolin-4-yl)phenyl)(6-methoxypyridazin-3-yl)methanol
2. M3814
3. Peposertib
1. Peposertib
2. 1637542-33-6
3. M3814
4. Msc2490484a
5. M-3814
6. Msc-2490484a
7. Gn429e725a
8. (s)-[2-chloro-4-fluoro-5-(7-morpholin-4-ylquinazolin-4-yl)phenyl]-(6-methoxypyridazin-3-yl)methanol
9. (s)-(2-chloro-4-fluoro-5-(7-morpholinoquinazolin-4-yl)phenyl)(6-methoxypyridazin-3-yl)methanol
10. Nedisertib [inn]
11. M-3814(nedisertib)
12. Peposertib [inn]
13. Nedisertib (deleted Inn)
14. Peposertib [who-dd]
15. Unii-gn429e725a
16. Gtpl9766
17. Chembl4297629
18. Schembl16235559
19. Bdbm315715
20. Ex-a1679
21. Mfcd31619234
22. Msc 2490484a
23. Nsc802822
24. Nsc816960
25. Us10172859, Example 136
26. Msc 2490484a [who-dd]
27. Nsc-802822
28. Nsc-816960
29. Example 136 [wo2014183850]
30. Hy-101570
31. Cs-0021723
32. 3-pyridazinemethanol, .alpha.-(2-chloro-4-fluoro-5-(7-(4-morpholinyl)-4-quinazolinyl)phenyl)-6-methoxy-, (.alpha.s)-
33. 3-pyridazinemethanol, Alpha-(2-chloro-4-fluoro-5-(7-(4-morpholinyl)-4-quinazolinyl)phenyl)-6-methoxy-, (alphas)-
| Molecular Weight | 481.9 g/mol |
|---|---|
| Molecular Formula | C24H21ClFN5O3 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 481.1316954 g/mol |
| Monoisotopic Mass | 481.1316954 g/mol |
| Topological Polar Surface Area | 93.5 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 662 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)