

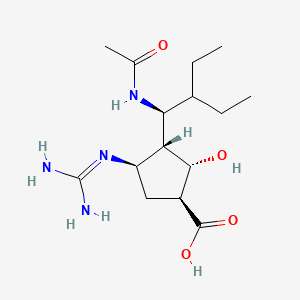

1. (1s,2s,3r,4r)-3-((1s)-1-acetamido-2-ethylbutyl)-4-((amino(imino)methyl)amino)-2-hydroxycyclopentanecarboxylic Acid Trihydrate
2. 3-(1'-acetylamino-2'-ethyl)butyl-4-((aminoimino)methyl)amino-2-hydroxycyclopentane-1-carboxylic Acid
3. Bcx 1812
4. Bcx-1812
5. Bcx1812
6. Peramivir Trihydrate
7. Rapivab
8. Rwj 270201
9. Rwj-270201
10. Rwj270201
1. 330600-85-6
2. Bcx-1812
3. 229614-55-5
4. Rapiacta
5. Rwj-270201
6. Rapivab
7. Peramivir Anhydrous
8. Chebi:85202
9. Bcx1812
10. Bcx 1812
11. Peramivir, (+/-)-
12. Rwj270201
13. (1s,2s,3s,4r)-3-[(1s)-1-acetamido-2-ethylbutyl]-4-(diaminomethylideneamino)-2-hydroxycyclopentane-1-carboxylic Acid
14. 229614-56-6
15. Rwj 270201
16. 9zs94hqo3b
17. Idl9q29886
18. (1s,2s,3r,4r)-3-((s)-1-acetamido-2-ethylbutyl)-4-guanidino-2-hydroxycyclopentanecarboxylic Acid
19. 330600-85-6 (free)
20. (1s,2s,3r,4r)-4-carbamimidamido-3-[(1s)-1-acetamido-2-ethylbutyl]-2-hydroxycyclopentane-1-carboxylic Acid
21. 3-(1-acetylamino-2-ethyl-butyl)-4-guanidino-2-hydroxy-cyclopentanecarboxylic Acid
22. 3-(1'-acetylamino-2'-ethyl)butyl-4-((aminoimino)methyl)amino-2-hydroxycyclopentane-1-carboxylic Acid
23. Bcz
24. Cyclopentanecarboxylic Acid, 3-[(1s)-1-(acetylamino)-2-ethylbutyl]-4-[(aminoiminomethyl)amino]-2-hydroxy-, (1s,2s,3r,4r)-
25. (+/-)-peramivir
26. Peramivir [usan:inn]
27. Unii-9zs94hqo3b
28. Peramiflu
29. Unii-idl9q29886
30. S-021812
31. Cyclopentanecarboxylic Acid, 3-((1s)-1-(acetylamino)-2-ethylbutyl)-4-((aminoiminomethyl)amino)-2-hydroxy-, (1s,2s,3r,4r)-
32. Peramivir [inn]
33. Peramivir [mi]
34. Schembl744373
35. Bdbm5024
36. Chembl139367
37. Schembl12795462
38. Dtxsid10904727
39. Bcpp000117
40. (1s,2r,3r,4r)-3-(1-acetamido-2-ethyl-butyl)-4-(diaminomethylideneamino)-2-hydroxy-cyclopentane-1-carboxylic Acid
41. Zinc3981610
42. Bdbm50181441
43. Hy-17015a
44. Mfcd09837902
45. Akos016007760
46. Akos037652424
47. Peramivir (+/-)-form [mi]
48. Cs-0638
49. Db06614
50. Dt-0029
51. Ncgc00346433-06
52. Ncgc00346433-07
53. (1s,2s,3r,4r)-3-((1s)-1-acetylamino-2-ethylbutyl))-4-((aminoiminomethyl)amino)-2-hydroxycyclopentane
54. (1s,2s,3r,4r)-3-((s)-1-acetamido-2-ethylbutyl)-4-guanidino-2-hydroxycyclopentane-1-carboxylic Acid
55. Ac-22715
56. Cyclopentanecarboxylic Acid, 3-((1r)-1-(acetylamino)-2-ethylbutyl)-4-((aminoiminomethyl)amino)-2-hydroxy-, (1r,2r,3s,4s)-rel-
57. P2892
58. W19713
59. Ab01566838_01
60. 600p856
61. A896277
62. Q412734
63. (-)-(1s,2s,3r,4r)-3-[(1s)-1-(acetylamino)-2-ethylbutyl]-4-{[amino(imino)methyl]amino}-2-hydroxycyclopentanecarboxylic Acid
64. (1s,2s,3r,4r)-3-((1s)-1-acetylamino-2-ethylbutyl)-4-((aminoiminomethyl)amino)-2-hydroxycyclopentanecarboxylic Acid
65. (1s,2s,3r,4r)-3-[(1s)-1-(acetylamino)-2-ethylbutyl]-4-[(aminoiminomethyl)amino]-2-hydroxycyclopentanecarboxylic Acid Trihydrate
66. (1s,2s,3r,4r)-3-[(1s)-1-(acetylamino)-2-ethylbutyl]-4-carbamimidamido-2-hydroxycyclopentanecarboxylic Acid
67. (1s,2s,3r,4r)-3-[(1s)-1-acetamido-2-ethyl-butyl]-4-guanidino-2-hydroxy-cyclopentanecarboxylic Acid
68. (1s,2s,3r,4r)-3-[(1s)-1-acetamido-2-ethylbutyl]-4-carbamimidamido-2-hydroxycyclopentane-1-carboxylic Acid
69. (1s,2s,3s,4r)-4-[(diaminomethylidene)amino]-3-[(1s)-1-acetamido-2-ethylbutyl]-2-hydroxycyclopentane-1-carboxylic Acid
70. Cyclopentanecarboxylic Acid, 3-((1s)-1-(acetylamino)-2-ethylbutyl)-4-((aminoiminomethyl)amino)-2-hydroxy-, (1r,2r,3s,4s)-rel-
| Molecular Weight | 328.41 g/mol |
|---|---|
| Molecular Formula | C15H28N4O4 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 328.21105539 g/mol |
| Monoisotopic Mass | 328.21105539 g/mol |
| Topological Polar Surface Area | 151 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 460 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than two days.
FDA Label
Alpivab is indicated for the treatment of uncomplicated influenza in adults and children from the age of 2 years.
Treatment of influenza
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
J05AH03
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AH - Neuraminidase inhibitors
J05AH03 - Peramivir
Peramivir is an inhibitor of influenza neuraminidase, preventing new virus particles from leaving infected cells.