


1. Celance
2. Ly-127,809
3. Ly-127809
4. Ly127,809
5. Ly127809
6. Mesylate, Pergolide
7. Parkotil
8. Pergolide
9. Permax
10. Pharken
1. 66104-23-2
2. Permax
3. Pergolide Methanesulfonate
4. Pergolide Mesylate Salt
5. Pergolide Mesilate
6. Pergolide (mesylate)
7. Ly127809
8. Pergolidemesylate
9. Ly-127809
10. Nsc-319773
11. Nsc-758442
12. Ly 127809
13. Mls000069837
14. Chebi:8021
15. 55b9hqy616
16. Mpe
17. Cpd000058504
18. Smr000058504
19. Pergolide Mesilate (jan)
20. Permax (tn)
21. Dsstox_cid_20583
22. Dsstox_rid_77029
23. Dsstox_gsid_40583
24. Pergolide Mesilate [jan]
25. Celance
26. Parkotil
27. Pharken
28. Chembl1275
29. Pergolide Mesylate [usan]
30. Sr-01000075395
31. Ncgc00017366-04
32. Nopar
33. Unii-55b9hqy616
34. (8b)-8-[(methylsulfanyl)methyl]-6-propylergoline Methanesulfonate
35. Sr-01000721840
36. Pergolide Mesylate [usan:usp]
37. (6ar,9r,10ar)-9-(methylsulfanylmethyl)-7-propyl-6,6a,8,9,10,10a-hexahydro-4h-indolo[4,3-fg]quinoline;methanesulfonic Acid
38. Prestwick_652
39. Cas-66104-23-2
40. Pergolide Monomesylate
41. Opera_id_186
42. Pergolide Mehanesulfonate
43. 8-beta-((methylthio)methyl)-6-propylergoline Methanesulfonate
44. 8beta-((methylthio)methyl)-6-propylergoline Monomethanesulfonate
45. 8-beta-((methylthio)methyl)-6-propylergoline Monomethane Sulfonate
46. Pergolide Mesylate (usp)
47. Pergolide Monomethanesulfonate
48. Schembl26920
49. Mls001148155
50. Mls001424320
51. Mls002222236
52. Spectrum1503269
53. Dtxsid6040583
54. Hms501g04
55. Ergoline, Methanesulfonate (1:1)
56. Hms1568l12
57. Hms1922o13
58. Hms2052h09
59. Hms2093c05
60. Hms2095l12
61. Hms2231f08
62. Hms3263e09
63. Hms3712l12
64. Hms3885k16
65. Pergolide Mesylate [vandf]
66. Pharmakon1600-01503269
67. Pergolide Mesilate [mart.]
68. Ex-a1334
69. Ly-141-b
70. Pergolide Mesilate [who-dd]
71. Pergolide Mesylate [usp-rs]
72. Tox21_110820
73. Tox21_500984
74. Ccg-39478
75. Hy-13720a
76. Nsc319773
77. Nsc758442
78. S4000
79. Akos015896681
80. Ergoline, 8-((methylthio)methyl)-6-propyl-, Monomethanesulfonate, (8beta)-
81. Ergoline, 8-beta-((methylthio)methyl)-6-propyl-, Methanesulfonate (1:1)
82. Tox21_110820_1
83. Ac-6874
84. Lp00984
85. Nc00428
86. Nsc 319773
87. Nsc 758442
88. Pergolide Mesylate [green Book]
89. Pergolide Methanesulfonate [mi]
90. Pergolide Mesylate [orange Book]
91. Ncgc00017366-08
92. Ncgc00094284-01
93. Ncgc00094284-02
94. Ncgc00094284-03
95. Ncgc00261669-01
96. Pergolide Mesilate [ep Monograph]
97. Pergolide Mesylate Salt, >=98%, Solid
98. As-76940
99. Pergolide Mesylate [usp Monograph]
100. Ergoline, (8.beta.)-, Monomethanesulfonate
101. Eu-0100984
102. D00502
103. D95035
104. P 8828
105. 104p232
106. Q-201548
107. Sr-01000075395-1
108. Sr-01000075395-3
109. Sr-01000721840-3
110. Q27107641
111. 8-[(methylthio)methyl]-6-propylergoline Methanesulfonate
112. (8beta)-8-[(methylsulfanyl)methyl]-6-propylergoline Methanesulfonate
113. 8-.beta.-[(methylthio)methyl]-6-propylergoline Methanesulfonate
114. Pergolide Mesilate, European Pharmacopoeia (ep) Reference Standard
115. (8beta)-8-[(methylsulfanyl)methyl]-6-propylergolin-6-ium Methanesulfonate
116. 8.beta.-((methylthio)methyl)-6-propylergoline Monomethanesulfonate
117. 8.beta.-((methylthio)methyl)-6-propylergoline Monomethanesulphonate
118. Pergolide Mesylate, United States Pharmacopeia (usp) Reference Standard
119. Ergoline, 8-((methylthio)methyl)-6-propyl-, Monomethanesulfonate, (8.beta.)-
120. Ergoline, 8-((methylthio)methyl)-6-propyl-, Monomethanesulphonate, (8.beta.)-
121. (6ar,9r,10ar)-9-((methylthio)methyl)-7-propyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline Methanesulfonate
122. (6ar,9r,10ar)-9-(methylthiomethyl)-7-propyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline Methanesulfonate
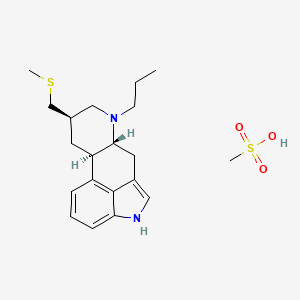
| Molecular Weight | 410.6 g/mol |
|---|---|
| Molecular Formula | C20H30N2O3S2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 410.16978517 g/mol |
| Monoisotopic Mass | 410.16978517 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)