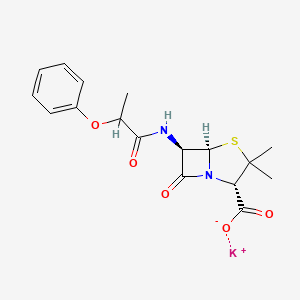

1. 3,3-dimethyl-7-oxo-6-(2-phenoxypropionamido)-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate
2. Broxil
3. Chemipen
4. Maxipen
5. Optipen
6. Penicillin, Phenoxyethyl
7. Pensig
8. Phenethicillin
9. Phenethicillin, (2s-(2alpha,5alpha,6beta(r*)))-isomer
10. Phenethicillin, (2s-(2alpha,5alpha,6beta))-isomer
11. Phenethicillin, (2s-(2alpha,5alpha,6beta,(s*)))-isomer
12. Phenethicillin, Monopotassium Salt, (2s-(2alpha,5alpha,6beta(r*)))-isomer
13. Phenethicillin, Monopotassium Salt, (2s-(2alpha,5alpha,6beta(s*)))-isomer
14. Phenethicillin, Monosodium Salt, (2s-(2alpha,5alpha,6beta))-isomer
15. Potassium Phenethicillin
16. Syncillin
1. Pheneticillin Potassium
2. Maxipen
3. Astracillin
4. Oralopen
5. Potassium Phenethicillin
6. 132-93-4
7. Phenethicillin Potassium Salt
8. Dramcillin S
9. Penicillin-152 Potassium
10. Phenethicillin K Salt
11. A-phenoxyethylpenicillinic Acid Potassium Salt
12. Chebi:31987
13. 70978wuk7c
14. 6-(a-phenoxypropionamido)penicillanic Acid Potassium Salt
15. Chemipen
16. Optipen
17. Syncillin
18. Broxil
19. Penova
20. Pensig
21. Monopotassium (2s,5r,6r)-3,3-dimethyl-7-oxo-6-(2-phenoxypropionamido)-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate
22. Alfacillin
23. Feneticilline
24. Synerpenin
25. Synthecillin
26. Synthecilline
27. Alticina
28. Bendralan
29. Brocsil
30. Penemve
31. Peniplus
32. Priospen
33. Semopen
34. Synapen
35. Triospen
36. Darcil
37. Alpha-oracillin
38. Penicillin Mv
39. Synthepen Tabl
40. Dramcillin-s
41. K Phenethicillin
42. Chemipen-c
43. Ro-cillin
44. Pheno-m-penicillin
45. Penicillin-152
46. Potassium;(2s,5r,6r)-3,3-dimethyl-7-oxo-6-(2-phenoxypropanoylamino)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
47. Pen 200
48. Phenoxyaethylpenicillin K-salz
49. Unii-70978wuk7c
50. Phenethicillin Potassium [jan]
51. Hsdb 3167
52. Brl 152
53. Syncillin (tn)
54. Synthepen (tn)
55. Ncgc00017019-01
56. 3,3-dimethyl-7-oxo-6-((1-oxo-2-phenoxypropyl)amino)-4-thia-1-azabicyclo(3.2.0)-heptane-2-carboxylic Acid Potassium Salt
57. Cas-132-93-4
58. Prestwick_142
59. Einecs 205-084-4
60. Phenethicillin Potassium [usp:jan]
61. Potassium (1-phenoxyethyl)penicillin
62. Phenethicillin-kalium
63. Potassium Methylphenoxymethylpenicillin
64. Phenoxyaethylpenicillin K-salz [german]
65. Potassium (alpha-phenoxyethyl)penicillin
66. Alpha-phenoxyethylpenicillin Potassium Salt
67. Dsstox_cid_25546
68. Dsstox_rid_80947
69. Dsstox_gsid_45546
70. Schembl34264
71. Spectrum1500643
72. Potassium 6-(alpha-phenoxypropionamido)penicillanate
73. Chembl2364720
74. Dtxsid5045546
75. Hms500l16
76. Phenethicillin Potassium (jp17)
77. Hms1570a08
78. Hms2092e16
79. Hms2097a08
80. Hms3714a08
81. Tox21_110739
82. Ccg-39275
83. Phenethicillin Potassium [mi]
84. Phenethicillin Potassium [hsdb]
85. Pheneticillin Potassium [mart.]
86. Pheneticillin Potassium [who-dd]
87. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-7-oxo-6-((1-oxo-2-phenoxypropyl)amino)-, Monopotassium Salt, (2s-(2alpha,5alpha,6beta))-
88. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-7-oxo-6-(2-phenoxypropionamido)-, Monopotassium Salt
89. D01178
90. Q27114742
91. Potassium 6beta-(2-phenoxypropanamido)-2,2-dimethylpenam-3alpha-carboxylate
92. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-7-oxo-6-((1-oxo-2-phenoxypropyl)amino)-, (2s-(2alpha,5alpha,6beta))-, Monopotassium Salt
93. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-7-oxo-6-((1-oxo-2-phenoxypropyl)amino)-, 92s-(2.alpha.,5.alpha.,6.beta.))-, Monopotassium Salt
| Molecular Weight | 402.5 g/mol |
|---|---|
| Molecular Formula | C17H19KN2O5S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 402.06517437 g/mol |
| Monoisotopic Mass | 402.06517437 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
THIS CONGENER OF PENICILLIN IS PHENOXYETHYL ANALOG OF PENICILLIN G. ... STABILITY IN ACIDIC MEDIA & BETTER ABSORPTION FROM GI TRACT...IS ITS SOLE ADVANTAGE IN COMPARISON TO PENICILLIN G. IT IS AVAIL ONLY FOR ORAL USE & HENCE IS NOT SUBSTITUTE FOR PARENTERAL PENICILLIN, WHEN SUCH THERAPY IS INDICATED. /PHENETHICILLIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1142
GINGIVOSTOMATITIS, PULMONARY INFECTIONS, & GENITAL DISEASE PRODUCED BY SYNERGISTIC ACTION OF FUSOBACTERIUM NUCLEATUM (FUSIFORM) & SPIROCHETES PRESENT IN RESPIRATORY TRACT ARE READILY TREATABLE WITH PENICILLIN. /PENICILLIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1156
PENICILLIN PROPHYLAXIS OF PROVEN VALUE. STREPTOCOCCAL INFECTIONS...RHEUMATIC FEVER RECURRENCES...GONORRHEA...SYPHILIS...SURGICAL PROCEDURES IN PATIENTS WITH VALVULAR HEART DISEASE. /PENICILLIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1157
...PENICILLIN CAN MARKEDLY ALTER NORMAL BACTERIAL FLORA OF MAN. ... SUPERIMPOSED INFECTION BY PENICILLIN-RESISTANT MICROORGANISM MAY DEVELOP... VERY HIGH CONCN OF PENICILLIN ARE NEUROTOXIC... /PENICILLIN/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1126
MECHANISM MOST COMMONLY INVOLVED IN PATHOGENESIS OF UNTOWARD EFFECTS FROM ANY TYPE OF PENICILLIN IS HYPERSENSITIZATION. ALLERGIC REACTIONS TO PENICILLINS VARY FROM 0.7 TO 10%... /PENICILLINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1082
IN SOME PERSONS...SUPRAINFECTION RESULTS FROM CHANGES IN FLORA. DERMATITIS INVOLVING PRIMARILY SCROTAL & INGUINAL SKIN...HAS BEEN OBSERVED... DRAMATIC EFFECT THAT MAY FOLLOW USE OF PENICILLIN IN SYPHILIS IS JARISCH-HERXHEIMER REACTION... /PENICILLINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1085
HIGH INCIDENCE OF CONTACT ALLERGIC REACTIONS WAS RECOGNIZED EARLY & TOPICAL APPLICATION OF PENICILLIN TO EYE HAS BEEN ESSENTIALLY ABANDONED CLINICALLY. /PENICILLIN/
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 793
For more Drug Warnings (Complete) data for PHENETHICILLIN POTASSIUM (7 total), please visit the HSDB record page.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
FOOD RETARDS ABSORPTION & THEREBY PROLONGS DURATION OF ACTION OF CMPD. PEAK PLASMA CONCN OCCUR ABOUT 1 HR AFTER ORAL ADMIN OF DRUG.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1142
GIVEN ORALLY, DRUG IS WELL ABSORBED & ESCAPES DESTRUCTION IN ACIDIC GASTRIC CONTENTS; DESPITE THIS FACT, INTESTINAL ABSORPTION IS INCOMPLETE. IN EQUAL ORAL DOSES, IT PRODUCES HIGHER PLASMA CONCN THAN DOES PENICILLIN G OR PENICILLIN V.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1142
PENICILLIN T/2 IN HUMAN SERUM INCR FROM ABOUT 25 MIN IN YOUNG ADULTS TO 2 HR IN ELDERLY SUBJECTS & IS ALSO MARKEDLY INCR BY DRUGS WHICH ARE ACTIVELY SECRETED BY KIDNEY TUBULES. SERUM PENICILLIN LEVELS IN NEW-BORN INFANTS ARE HIGHER & MORE PROLONGED THAN IN CHILDREN & ADULTS AFTER EQUIV DOSES. /PENICILLIN/
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 167
...RAPIDLY ELIMINATED FROM BODY, MAINLY BY KIDNEY BUT IN SMALL PART IN BILE & BY OTHER CHANNELS. ... CLEARANCE VALUES ARE CONSIDERABLY LOWER IN NEONATES & INFANTS, BECAUSE OF INCOMPLETE DEVELOPMENT OF RENAL FUNCTION... /PENICILLIN G/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1071
SIGNIFICANT AMT APPEAR IN LIVER, BILE, KIDNEY, SEMEN, LYMPH, & INTESTINE. ... PENICILLIN DOES NOT READILY ENTER CSF WHEN MENINGES ARE NORMAL. /PENICILLIN G/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1070
PENICILLIN T/2 IN HUMAN SERUM INCR FROM ABOUT 25 MIN IN YOUNG ADULTS TO 2 HR IN ELDERLY SUBJECTS. /PENICILLIN/
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 167
...ACT TO INHIBIT SYNTH OF COMPONENTS OF BACTERIAL CELL WALL... /PENICILLIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1135
The penicillins and their metabolites are potent immunogens because of their ability to combine with proteins and act as haptens for acute antibody-mediated reactions. The most frequent (about 95 percent) or "major" determinant of penicillin allergy is the penicilloyl determinant produced by opening the beta-lactam ring of the penicillin. This allows linkage of the penicillin to protein at the amide group. "Minor" determinants (less frequent) are the other metabolites formed, including native penicillin and penicilloic acids. /Penicillins/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 953