
1. Ap-la-day
2. Ex-lax
3. Feen-a-mint
4. Laxatone
5. Laxettes
6. Modane
7. Thalinol
1. 77-09-8
2. Phthalimetten
3. Euchessina
4. Phthalin
5. Espotabs
6. Phenolax
7. Koprol
8. Laxogen
9. Trilax
10. Purga
11. Spulmako-lax
12. Feen-a-mint Gum
13. Purgen
14. 3,3-bis(4-hydroxyphenyl)phthalide
15. 3,3-bis(4-hydroxyphenyl)-1(3h)-isobenzofuranone
16. Alophen
17. Doxidan
18. Dihydroxyphthalophenone
19. Phillips Gelcaps
20. 1(3h)-isobenzofuranone, 3,3-bis(4-hydroxyphenyl)-
21. 3,3-bis(p-hydroxyphenyl)phthalide
22. Fenolftalein
23. Phenolphtaleine
24. Phenophthalein
25. 3,3-bis(4-hydroxyphenyl)isobenzofuran-1(3h)-one
26. 3,3-bis(4-hydroxyphenyl)-2-benzofuran-1-one
27. Nci-c55798
28. Feen-a-mint Laxative Mints
29. 3,3-bis(4-hydroxyphenyl)-2-benzofuran-1(3h)-one
30. Nsc 10464
31. Component Of Agoral
32. 3,3-bis(4-hydroxyphenyl)-1,3-dihydro-2-benzofuran-1-one
33. Phenolphthalein,white
34. Phthalide 3,3,-bis(p-hydroxyphenyl)-
35. Mfcd00005913
36. Chembl63857
37. Mls000069592
38. 6qk969r2if
39. Correctol
40. Dtxsid0021125
41. Medilax
42. Chebi:34914
43. Colax
44. Laxin
45. Femilax
46. Lax-pills
47. Nsc-10464
48. Evac-q-kwik
49. Evac-q-kit
50. Evac-u-gen
51. Component Of Correctol
52. Phenolphthalein (inn)
53. Ncgc00018200-07
54. Smr000059015
55. Agoral
56. Fenolftalein [czech]
57. Dsstox_cid_1125
58. Dsstox_rid_75956
59. Dsstox_gsid_21125
60. Phenolphthalein [inn]
61. Component Of Feen-a-mint Pills
62. Fenolftaleina [inn-spanish]
63. Phenolphtaleine [inn-french]
64. Phenolphthaleinum [inn-latin]
65. Fenolftaleina
66. Phenolphthaleinum
67. Phthalide 3,-bis(p-hydroxyphenyl)-
68. Alpha-di(p-hydroxyphenyl)phthalide
69. Cas-77-09-8
70. Phenolphthalein Solution, Alcoholic 1.0%
71. Ccris 6266
72. Wln: T56 Bvo Dhj D- D-/r Dq 2
73. Hsdb 4161
74. 1(3h)-isobenzofuranone,3-bis(4-hydroxyphenyl)-
75. 3, 3-bis(p-hydroxyphenyl)-1(3h)-isobenzofuranone
76. Einecs 201-004-7
77. Brn 0284423
78. Phenolphtalein
79. Unii-6qk969r2if
80. Ai3-09081
81. Phenolphthalein [usp:inn:ban]
82. Fgt
83. Doxan (salt/mix)
84. Agoral (salt/mix)
85. Modane (tn)
86. Phenolphthalein,(s)
87. Laxcaps (salt/mix)
88. Phenolphthalein,yellow
89. Yellow Phenolphthalein
90. Phenolphthalein Solution
91. Spectrum_001077
92. Opera_id_1337
93. Spectrum2_001279
94. Spectrum3_000888
95. Spectrum4_000982
96. Spectrum5_001268
97. Modane Plus (salt/mix)
98. 3,3-bis(4-hydroxyphenyl)isobenzofuran-1-one
99. Ec 201-004-7
100. Phenolphthalein Acs Reagent
101. Phenolphthalein Solution R1
102. Phenolphthalin [mi]
103. Cid_4764
104. Alpha-(p-hydroxyphenyl)-alpha-(4-oxo-2,5-cyclohexadien-1-ylidene)-o-toluic Acid
105. Phenolphthalein [mi]
106. Phenolphthalein, Acs Reagent
107. Schembl27670
108. Bspbio_002518
109. Kbiogr_001383
110. Kbioss_001557
111. Phenolphthalein, Ph Indicator
112. Phenolphthalein, White
113. 5-18-04-00188 (beilstein Handbook Reference)
114. Mls001148397
115. Bidd:er0202
116. Divk1c_000929
117. Phenolphthalein [hsdb]
118. Phenolphthalein [iarc]
119. Phenolphthalein [inci]
120. Spectrum1500480
121. Phenolphthalein Paper, Dab 6
122. Spbio_001278
123. Phenolphthalein [vandf]
124. 3,3-bis(4-hydroxyphenyl)-3-hydroisobenzofuran-1-one
125. Phenolphthalein [mart.]
126. Phenpolphthalein [vandf]
127. Phenolphthalein [who-dd]
128. Bcbcmap01_000174
129. Hms502o11
130. Kbio1_000929
131. Kbio2_001557
132. Kbio2_004125
133. Kbio2_006693
134. Kbio3_001776
135. Phenolphthalein Solution, 0.1 N
136. Ninds_000929
137. Hms1920h04
138. Hms2091p06
139. Hms2236i09
140. Hms3374p06
141. Pharmakon1600-01500480
142. Component Of Correctol (salt/mix)
143. Amy37148
144. Hy-d0211
145. Nsc10464
146. Zinc3831317
147. Phenolphthalein, P.a., Acs Reagent
148. Tox21_110838
149. Tox21_202219
150. Tox21_300282
151. Bbl002030
152. Bdbm50077844
153. Ccg-39112
154. Nsc215214
155. Nsc757271
156. Phenolphthalein Ph Indicator Solution
157. Phenolphthalein,white [vandf]
158. S5395
159. Stk029876
160. Phenolphthalein [ep Monograph]
161. Akos000493033
162. Tox21_110838_1
163. Cs-8152
164. Db04824
165. Nsc-215214
166. Nsc-757271
167. Phenolphthalein, 0.5% W/v In Alcohol
168. Idi1_000929
169. Smp1_000235
170. Ncgc00018200-01
171. Ncgc00018200-02
172. Ncgc00018200-03
173. Ncgc00018200-04
174. Ncgc00018200-05
175. Ncgc00018200-06
176. Ncgc00018200-08
177. Ncgc00018200-09
178. Ncgc00018200-10
179. Ncgc00018200-12
180. Ncgc00023694-03
181. Ncgc00023694-04
182. Ncgc00023694-05
183. Ncgc00023694-06
184. Ncgc00023694-07
185. Ncgc00254039-01
186. Ncgc00259768-01
187. Ac-14431
188. Bp-30100
189. Vs-01000
190. Phenolphthalein 100 Microg/ml In Methanol
191. Sbi-0051481.p003
192. Component Of Feen-a-mint Pills (salt/mix)
193. Eu-0082600
194. Ft-0659094
195. P0094
196. P0700
197. P0701
198. P0702
199. 3,3-bis(4-hydroxyphenyl)-2-benzofuran-1-on
200. En300-92962
201. Phenolphthalein, 2% Solution In 95% Ethanol
202. Phenolphthalein, Saj First Grade, >=97.0%
203. .alpha., .alpha.-di(p-hydroxyphenyl)phthalide
204. 3,3-bis (4-hydroxyphenyl)-2-benzofuran-1-on
205. D05456
206. H12020
207. Phenolphthalein, Jis Special Grade, >=98.0%
208. Ab00052070_15
209. Q187921
210. Sr-01000000112
211. Q-201555
212. Sr-01000000112-2
213. 3,3-bis(4-hydroxyphenyl)-2-benzofuran-1(3h)-one #
214. 3,3-bis-(4-hydroxy-phenyl)-3h-isobenzofuran-1-one
215. Brd-k19227686-001-02-0
216. Brd-k19227686-001-12-9
217. Phenolphthalein Solution, 1 % (w/v), Ph 7.8-10.0
218. Z57233591
219. F0921-4309
220. Phenolphthalein (263 Degrees C) Melting Point Standard
221. Phenolphthalein Solution, 0.04 % (w/v), Ph 7.8-10.0
222. Phenolphthalein Solution, 0.1 % (w/v), Ph 7.8-10.0
223. Phenolphthalein Solution, 0.5 Wt. % In Ethanol: Water (1:1)
224. Phenolphthalein, United States Pharmacopeia (usp) Reference Standard
225. Phenolphthalein, Acs Reagent, Reag. Ph. Eur., Indicator, 98-102% (calc. To The Dried Substance), S. No.: 879
226. Phenolphthalein, Puriss., Meets Analytical Specification Of Ph??eur., Bp, 98-101% (calc. To The Dried Substance)
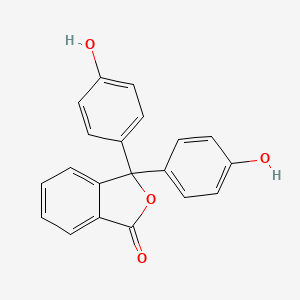
| Molecular Weight | 318.3 g/mol |
|---|---|
| Molecular Formula | C20H14O4 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 318.08920892 g/mol |
| Monoisotopic Mass | 318.08920892 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 438 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cathartics; Indicators and Reagents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...Has...been widely employed as cathartic. ... /it/ is avail...in numerous proprietary preparations.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1046
Since ... laxative effects are not usually produced in less than 6 hr after oral administration, they are often taken at bedtime, to produce their effect next morning. Because of adverse effects, use of these agents should be limited to 10 consecutive days. /diphenylmethane derivatives/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1046
(VET): Has been used as a laxative.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1300
Cathartic drug in laxatives; acid-base indicator
SRI
Use of phenolphthalein by women during breast-feeding may cause diarrhea in their infants.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 399 (2000)
Patients should be warned of possible coloring of urine and feces.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 923
Used for over a century as a laxative.
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AB - Contact laxatives
A06AB04 - Phenolphthalein
Up to 15% of therapeutic dose of phenolphthalein is absorbed and eliminated by kidney, most of it in conjugated form.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1011
Urine becomes pink or red if it is sufficiently alkaline (pH 7or more). Some absorbed drug is also exreted in the bile, and the resulting enterohepatic cycle may contribute to prolongation of laxative effect.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1011
Biliary excretion of metabolites of...phenolphthalein...in rats was shown...to be increased by pre-treatment with hepatic-microsomal-enzyme inducers, and to be decreased by enzyme inhibitors after dosing with parent compound, but no effect was observed after dosing with metabolites.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 436
Phenolphthalein glucuronide (I) excreted more rapidly than phenolphthalein (II) following iv injection, suggesting that uptake of (I) from blood imposes rate limitation which outweighs the lack of conjugation before excretion.
Clark A et al; J PHARM PHARMACOL 30 (6): 382 (1978)
For more Absorption, Distribution and Excretion (Complete) data for PHENOLPHTHALEIN (14 total), please visit the HSDB record page.
Phenolphthalein that had undergone conjugation, but no unmetabolized phenolphthalein, was excreted into breast milk in concentrations up to 1.0 ug/mL after a single 200-800 mg dose in 22 lactating women. Bowel movements occurred in 16 of the women after the dose, but none of the nursing infants had diarrhea.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 683
Yields phenolphthalein-beta-d-glucuronide in rat, in mouse. /from table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. 8
Phenolphthalein is absorbed in the intestine and is almost completely converted to its glucuronide during extensive first-pass metabolism in the intestinal epithelium and liver via uridine diphosphate glucuronosyltransferase (UDPGT) in rodents and dogs. In guinea-pigs, small amounts of sulfate-conjugated metabolites have been detected in isolated mucosal sheets originating in the jejunum and colon. Fecal excretion is the major route of elimination of phenolphthalein in rats, while in mice both urinary and fecal elimination are important. The metabolites identified in urine and feces are phenolphthalein glucuronide, phenolphthalein sulfate and phenolphthalein hydroxide.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 399-400 (2000)
...The major metabolite was PHTH glucuronide. Three minor metabolites were detected. A sulfate conjugate and and a hydroxylated metabolite were identified by comparison of retention times and 1H NMR and/or mass spectra with synthetic standards. A diglucuronide conjugate was tentatively identified. Biliary elimination was extensive in rats (35% of dose within 6 hr); the only product detected in bile was phenolphthalein glucuronide
PMID:9579019 Griffin RJ et al; Toxicol Sci 42 (2): 73-81 (1998)
Classical compartmental pharmacokinetics model developed to describe systemic blood concentration-time profile of phenolphthalein following single iv bolus injection; used to simulate 24-hr time course of blood concentration. Indication that long t/2 are artifacts of recirculation.
PMID:38070 Colburn W et al; DRUG METAB DISPOS 7 (2): 100 (1979)
Stimulant drugs alter fluid and electrolyte absorption, producing net intestinal fluid accumulation and laxation. Increased concentrations of cyclic 3'-5'-adenosine monophosphate (CAMP) occurring in clonic mucosal cells may alter the permeability of these cells leading to net fluid accumulation and laxative action. Phenolphthalein also acts directly or reflexly to increase the the activity of the small intestine. Phenolphthalein acts mainly on the colon about 6 hours after ingestion.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1011
When taken orally, it is thought to be dissolved by intestinal juices and bile and to stimulate intestinal musculature, chiefly that of colon.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 742
Phenolphthalein increased intestinal fluid volume in rat colon in situ, apparently via stimulation of prostaglandin E biosynthesis in colon.
PMID:631275 Beubler E et al; EXPERIENTIA 34 (3): 386 (1978)
Water absorption from intestines of 6 pt with ileostomies and from rats was measured after administration of phenolphthalein. Results indicate that some laxative effects result from inhibition of water absorption in large and small intestines.
PMID:717350 Saunders D et al; AM J DIG DIS 23 (OCT): 909 (1978)
.... A catechol metabolite of PT, hydroxyphenolphthalein (PT-CAT), was recently identified and may be the molecular species responsible for at least part of the toxicity/carcinogenicity of PT. We hypothesize that PT-CAT inhibits the enzyme catechol-O-methyltransferase (COMT) and therefore potentiates genotoxicity by either PT-CAT itself or the endogenous catechol estrogens (CEs) in susceptible tissues. The present studies were conducted to determine the effects of PT treatment and PT-CAT itself on the COMT-mediated metabolism of 4- and 2-hydroxyestradiol both in vitro and in vivo. Female mice were treated with PT (50 mg/kg/d) for 21 days and then euthanized. PT-CAT concentration in urine reached plateau levels by 7 days of exposure. An O-methylated metabolite of PT-CAT was detected in feces. In vitro experiments demonstrated that PT treatment resulted in an increase in free CEs, which are normally cleared by COMT and a concurrent decrease in the capacity of hepatic catechol clearance by COMT. In vitro, PT-CAT was a substrate of COMT, with kinetic properties within the range measured with endogenous substrates. PT-CAT was an extremely potent mixed-type inhibitor of the O-methylation of the catechol estrogens, with 90-300 nM IC50s.
PMID:10637136 Garner CE, et al; Toxicol Appl Pharmacol 162(2): 124-31 (2000)