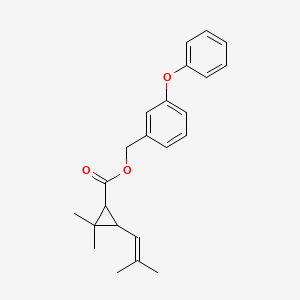

1. (3-phenoxyphenyl)methyl Cis,trans-(+)-2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
2. D-phenothrin
3. Phenothrin, (1r-cis)-isomer
4. Phenothrin, (1r-trans)-isomer
5. Phenothrin, (1s-cis)-isomer
6. Phenothrin, (1s-trans)-isomer
7. Phenothrin, (cis-(+-))-isomer
8. Phenothrin, (trans-(+-))-isomer
9. S-2539
10. Sumithrin
1. 26002-80-2
2. Sumithrin
3. Phenoxythrin
4. Phenothrine
5. Pibutin
6. Sumitrin
7. Duet
8. Anchimanaito 20s
9. Solo (insecticide)
10. Multicide 2154
11. Fenotrina
12. S 2539 (pesticide)
13. 188023-86-1
14. 3-phenoxybenzyl Chrysanthemate
15. Hegor Antipoux
16. D-phenotrhin
17. Oms 1809
18. Oms 1810
19. Pt 515
20. (3-phenoxyphenyl)methyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate
21. Nsc-758668
22. S-2539f
23. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, (3-phenoxyphenyl)methyl Ester
24. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-,(3-phenoxyphenyl)methyl Ester
25. Wellcide
26. Chebi:34916
27. M-phenoxybenzyl 2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
28. Phenothrin (inn)
29. 2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylic Acid M-phenoxybenzyl Ester
30. 3-phenoxybenzyl 2,2-dimethyl-3-(2-methylprop-1-en-1-yl)cyclopropanecarboxylate
31. 3-phenoxybenzyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
32. 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylic Acid (3-phenoxyphenyl)methyl Ester
33. Phenothrin 10 Microg/ml In Isooctane
34. (-)-trans-phenothrin
35. Ncgc00094561-01
36. Phenothrin [inn]
37. 707484x33x
38. Benzyl Alcohol, M-phenoxy-, 2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
39. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methylpropenyl)-, M-phenoxybenzyl Ester
40. Dsstox_cid_12688
41. Dsstox_rid_79033
42. Dsstox_gsid_32688
43. Fenotrina [spanish]
44. Phenothrine [french]
45. Phenothrinum [latin]
46. Caswell No. 652b
47. Phenothrinum
48. Phenothrin [inn:ban]
49. Phenothrine [iso-french]
50. Phenothrin [iso]
51. Cas-26002-80-2
52. Ccris 2502
53. Multicide Concentrate F-2271
54. Hsdb 3922
55. Phenothrin [bsi:iso]
56. Einecs 247-404-5
57. S-2539
58. Ent 27 972
59. Epa Pesticide Chemical Code 069005
60. Phonothrin
61. Delta-(cis-trans)-phenothrin
62. Ai3-29062
63. 3-phenoxybenzyl (+-)-cis-trans-chrysanthemate
64. (r)-phenothrin
65. 3-phenoxybenzyl (1rs)-cis,trans-chrysanthemate
66. 3-phenoxybenzyl 2-dimethyl-3-(methylpropenyl)cyclopropanecarboxylate
67. Unii-707484x33x
68. Hegor Antipoux (tn)
69. Spectrum_001981
70. Phenothrin [mi]
71. Specplus_000796
72. Phenothrin [jan]
73. Spectrum3_001204
74. Spectrum4_000583
75. Spectrum5_000644
76. Phenothrin [hsdb]
77. Phenothrin [mart.]
78. (3-phenoxyphenyl)methyl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
79. 3-phenoxybenzyl (1rs)-cis,trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
80. 3-phenoxybenzyl (1rs,3rs;1rs,3sr)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
81. Phenothrin [who-dd]
82. Schembl74649
83. Bspbio_002588
84. Kbiogr_001046
85. Kbioss_002547
86. Mls004712075
87. Divk1c_006892
88. Spectrum1504098
89. Delta -(cis-trans)-phenothrin
90. Chembl1322884
91. Dtxsid7032688
92. Kbio1_001836
93. Kbio2_002538
94. Kbio2_005106
95. Kbio2_007674
96. Kbio3_002088
97. Hms1922b17
98. Pharmakon1600-01504098
99. Bba00280
100. Hy-b1072
101. Tox21_111297
102. Tox21_301570
103. Ccg-38960
104. Nsc758668
105. Akos015914551
106. Phenothrin 100 Microg/ml In Methanol
107. Tox21_111297_1
108. Cs-4628
109. Db13717
110. Nsc 758668
111. M-phenoxybenzyl (+-)-cis,trans-2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
112. Phenothrin 1000 Microg/ml In Methanol
113. Ncgc00094561-02
114. Ncgc00094561-03
115. Ncgc00094561-04
116. Ncgc00094561-06
117. Ncgc00255299-01
118. As-76801
119. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 3-(phenoxyphenyl)methyl Ester
120. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 3-(phenoxyphenyl)methyl Ester, Cis,trans-(+/-)-
121. Smr001563205
122. 3-phenoxybenzyl-d-cis.trans-chrysanthemate
123. Sbi-0051922.p002
124. Db-046812
125. Db-046822
126. Ft-0630520
127. Ft-0630521
128. Sw220054-1
129. Phenothrin, Pestanal(r), Analytical Standard
130. D08357
131. S 2539
132. Ab00052445_02
133. Ab00052445_03
134. 002p802
135. A818137
136. Q999813
137. Sr-01000872757
138. J-016219
139. Sr-01000872757-1
140. Brd-a22106989-001-01-9
141. Brd-a22106989-001-02-7
142. (3-phenoxyphenyl)methyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
143. 2,2-dimethyl-3-(2-methylpropenyl-1)cyclopropancarbonic Acid, 3-phenoxybenzyl Ester
144. 3-phenoxybenzyl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate #
145. 3-phenoxybenzyl 2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
146. 3-phenoxyphenylmethyl 2,2-dimethyl- 3-(2-methyl-1-propenyl)cyclopropanecarboxylate
147. Cyclopropanecarboxylic Acid,2,2-dimethyl-3-(2-methylpropen-1-yl), 3-phenoxybenzyl Ester
148. 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylic Acid (3-phenoxy-phenyl)methyl Ester
149. M-phenoxybenzyl (+/-)-cis,trans-2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate
| Molecular Weight | 350.4 g/mol |
|---|---|
| Molecular Formula | C23H26O3 |
| XLogP3 | 6.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 350.18819469 g/mol |
| Monoisotopic Mass | 350.18819469 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 512 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pyrethrins with piperonyl butoxide are used for topical treatment of pediculosis (lice infestations). Combinations of pyrethrins with piperonyl butoxide are not effective for treatment of scabies (mite infestations). Although there are no well-controlled comparative studies, many clinicians consider 1% lindane to be pediculicide of choice. However, some clinicians recommend use of pyrethrins with piperonyl butoxide, esp in infants, young children, & pregnant or lactating women ... . If used correctly, 1-3 treatments ... are usually 100% effective ... Oil based (eg, petroleum distillate) combinations ... produce the quickest results. ... For treatment of pediculosis, enough gel, shampoo, or solution ... should be applied to cover affected hair & adjacent areas ... After 10 min, hair is ... washed thoroughly ... treatment should be repeated after 7-10 days to kill any newly hatched lice. /Pyrethrins/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3203
One hundred and one subjects with head louse infestation were entered into two separate studies, in which a phenothrin aqueous/alcoholic lotion was compared to a carbaryl lotion and a malathion lotion. Fifty subjects were treated with a single application of the phenothrin lotion, 28 with the carbaryl lotion and 23 with the malathion lotion. In the comparative study of the phenothrin and malathion lotions an inspection on the day following treatment showed no live lice remained, but that six of the subjects treated with malathion lotion still had evidence of viable eggs (p < 0.05). In one subject viable eggs were still evident at two weeks post-treatment. There were no cases, however, of live lice or viable eggs at four weeks post-treatment. Mild cutaneous side-effects were reported in five subjects, the incidence of which was not significantly different by treatment group. One subject in the phenothrin and carbaryl lotion comparative study had evidence of live lice at one week post-treatment with phenothrin lotion. This subject received no further treatment and was clear of both live lice and viable eggs at subsequent visits. A separate case of live lice infestation was found at two weeks post-treatment in a subject treated with phenothrin lotion and at four weeks post-treatment in two subjects treated with carbaryl lotion. As these subjects were free of live lice infestation at previous follow-up visits it was highly probable that these were cases of re-infestation from another source.
PMID:1903813 Doss S et al; J R Soc Health 111 (2): 47-50 (1991)
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
P - Antiparasitic products, insecticides and repellents
P03 - Ectoparasiticides, incl. scabicides, insecticides and repellents
P03A - Ectoparasiticides, incl. scabicides
P03AC - Pyrethrines, incl. synthetic compounds
P03AC03 - Phenothrin
Dermal adsorption of (+)trans- and (+)cis-phenothrin into body of male rats from dust or emulsifiable concentrate (EC) was estimated to be 3-7% and 8-17%. The rate of absorption was 4-5 times faster with EC than with dust and T/2 in blood was 2-3 times longer.
Kaneko H et al; Nippon Noyaku Gakkaishi (J Pestic Sci) 6 (2): 169-82 (1981)
(14)C-phenothrin labeled at the hydroxymethyl group of the alcohol moiety was orally admin at ... 200 mg/kg to male Sprague-Dawley rats. Absorption and elimination was rapid. About 60% of radioactivity was eliminated in urine and 40% in feces in 3 days. In addn to phenothrin, 3-phenoxybenzyl alcohol and 3-phenoxybenzoic acid were found in brain, liver, kidney, and blood. Unidentified water and ether solubles were also present.
Menzie, C.M. Metabolism of Pesticides, Update II. U.S. Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 2l2. Washington, DC: U.S. Government Printing Office, 1978., p. 238
Dermal adsorption of (+)trans- and (+)cis-phenothrin into body of male rats from dust or emulsifiable concentrate (EC) was estimated to be 3-7% and 8-17%. Rate of absorption was 4-5 times faster with EC than with dust. Amount absorbed through skin was almost completely excreted into urine and feces within 6 days. When admin once orally, at rate of 2 mg/kg (either isomer), about 96% of dose was recovered in excreta during following 6 days. A larger amt of (+)cis-isomer was excreted in feces than (+)trans-isomer and a larger amt of (+)trans-isomer was excreted in urine than (+)-cis-isomer.
Kaneko H, et al; Nippon Noyaku Gakkaishi (J Pestic Sci) 6 (2): 169-82 (1981)
The tissue residues in rats 7 days after a single oral dose of (14)C-(1R,cis)- or (14)C-(1R,trans)-phenothrin at 10 mg/kg body weight were generally very low although the fat showed somewhat higher residue levels (1-2.5 mg/kg). Similarly, high 14C residue levels (up to 23 mg/kg) were found in the fat, 7 days after a single oral dose of the [1R,cis] isomer at 200 mg/kg body weight.
WHO; Environmental Health Criteria 96: Phenothrin p.29 (1990)
For more Absorption, Distribution and Excretion (Complete) data for PHENOTHRIN (13 total), please visit the HSDB record page.
(14)C-Phenothrin ... was orally admin at ... 200 mg/kg to male Sprague-Dawley rats. ... Urine contained low levels of 3-phenoxybenzoic acid and its glycine conjugate and some ether and water sol material. In addn ... 3-(4'-hydroxyphenoxy)benzoic acid was present and accounted for 42.3% of radioactivity ... This cmpd was ... major metab in feces but accounted for only 11.9% of ... radioactivity. In addn to unchanged phenothrin and unidentified water and ether solubles, feces contained 3-phenoxybenzoic acid and the glycine conjugate. 3-phenoxybenzyl alcohol was not observed in urine or feces.
Menzie, C.M. Metabolism of Pesticides, Update II. U.S. Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 2l2. Washington, DC: U.S. Government Printing Office, 1978., p. 238
Dermal and oral admin of (+)trans- and (+)cis-phenothrin to male rats from dust or emulsifiable concentrate produced nearly the same metabolites. Major metabolites from (+)trans-isomer were 3-phenoxybenzoic acid and its glycine conjugate and (3,4'-hydroxyphenoxy)benzoic acid and its sulfate. The cis-isomer gave larger amounts of ester metabolites.
Kaneko H et al; Nippon Noyaku Gakkaishi (J Pestic Sci) 6 (2): 169-82 (1981)
When [1R,trans]-phenothrin was given to rats at 4, 10, or 200 mg/kg body weight (oral single dose) or 4 mg/kg body weight (repetitive oral dose for 14 days), the sulfate conjugate of 4'-OH-phenoxy benzoic acid was predominant, accounting for 28, 43, 28, and 55%, respectively, of the dose. In addition, phenoxy benzoic acid (4, 10, 5, and 6%), its glycine conjugate (1,3,2, and 2%) and glucuronide (2,3,1, and 3%), and free 4'-OH-phenoxybenzoic acid (2,11,3, and 3%) were found. The sulfate conjugate of 3-(2'-hydroxyphenoxy)benzoic acid (2'-OH-PBacid) was also found as a minor metabolite.
WHO; Environmental Health Criteria 96: Phenothrin p.29 (1990)
When Sprague Dawley rats were administered a single oral dose of [1R,trans]-phenothrin at 4 or 200 mg/kg body weight level or given an oral dose of 4 mg/kg body weight per day for 14 days, unmetabolized compound was found in the feces (44-45, 44-60, and 14-16% of the dose, respectively). An ester-form metabolite, the 4'-hydroxy phenoxy benzoic acid derivative of trans-phenothrin, was also detected (0.4-0.6%).
WHO; Environmental Health Criteria 96: Phenothrin p.30 (1990)
For more Metabolism/Metabolites (Complete) data for PHENOTHRIN (14 total), please visit the HSDB record page.
Following absorption through the chitinous exoskeleton of arthropods, pyrethrins stimulate the nervous system, apparently by competitively interfering with cationic conductances in the lipid layer of nerve cells, thereby blocking nerve impulse transmissions. Paralysis and death follow. /Pyrethrins/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3203
Some synthetic pyrethroids given intravenously to rats cause either tremor (T-syndrome) or choreoathetosis with salivation (CS-syndrome). However, d-phenothrin (>600 mg/kg body weight) injected intravenously into the lateral tail vein caused neither T-syndrome nor CS syndrome, due to its very low acute toxicity. From a study involving intracerebral dosing with [1R,cis]- or [1R, trans]-phenothrin in mice, both compounds were classified as Type I pyrethroids based on the occurrence of tremors and on neurophysiological studies in cockroach cercal sensory nerves.
WHO; Environmental Health Criteria 96: Phenothrin p.43 (1990)
The effects of 4 different pyrethroid insecticides on sodium channel gating in internally perfused, cultured mouse neuroblastoma cells (N1E-115) were studied using the suction pipette, voltage clamp technique. Pyrethroids increased the amplitude of the sodium current, sometimes by more than 200%. Activation of the sodium current occurred at more hyperpolarized potentials than under control conditions. The declining phase of the sodium current during depolarization was markedly slowed down and after repolarization of the membrane a large, slowly decaying sodium tail current developed. Pyrethroids did not affect the sodium current reversal potential, steady-state sodium inactivation or recovery from sodium channel inactivation. The amplitude of the pyrethroid-induced slow tail current was always proportional to the sodium current at the end of the preceding depolarizing pulse. The rate of decay of the slow tail current strongly depended on pyrethroid structure and increased in the order deltamethrin, cyphenothrin, fenfluthrin and phenothrin. The rate of decay further depended on membrane potential and temperature. Below -85 m V the instantaneous current-voltage relationship of the slow tail current showed a negative slope conductance. The tail current decayed more slowly at low temperatures. Arrhenius plots indicated that the relaxation of open sodium channels to a closed state involved a higher energy barrier for pyrethroid-affected than for normal channels. The energy barrier was higher after deltamethrin than after the non-cyano pyrethroid fenfluthrin. It is concluded that in mammalian neuronal membrane pyrethroids selectively reduce the rate of closing of sodium channels both during depolarization and after repolarization of the nerve membrane.
PMID:2449265 Ruigt GS et al; Brain Res 437 (2): 309-22 (1987)
The synthetic pyrethroids delay closure of the sodium channel, resulting in a sodium tail current that is characterized by a slow influx of sodium during the end of depolarization. Apparently the pyrethroid molecule holds the activation gate in the open position. Pyrethroids with an alpha-cyano group (e.g., fenvalerate) produce more prolonged sodium tail currents than do other pyrethroids (eg, permethrin, bioresmethrin). The former group of pyrethroids causes more cutaneous sensations than the latter. /Synthetic pyrethroids/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 1081
For more Mechanism of Action (Complete) data for PHENOTHRIN (11 total), please visit the HSDB record page.