
1. Falithrom
2. Liquamar
3. Marcoumar
4. Marcumar
5. Phenprocoumalol
6. Phenprocoumarol
7. Phenprogramma
8. Phenylpropylhydroxycumarinum
1. 435-97-2
2. Phenprocoumarol
3. Phenprocoumarole
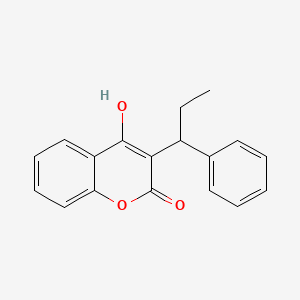
4. 4-hydroxy-3-(1-phenylpropyl)-2h-chromen-2-one
5. Liquamar
6. Marcumar
7. Fencumar
8. Marcoumar
9. Falithrom
10. Phenprocumone
11. Phenprocoumone
12. Fenprocumone
13. Phenprocoumonum
14. Fenprocumone [dcit]
15. 3-(1-phenylpropyl)-4-hydroxycoumarin
16. 4-hydroxy-3-(1-phenylpropyl)-2h-1-benzopyran-2-one
17. 3-(1'-phenyl-propyl)-4-oxycoumarin
18. Ro 1-4849
19. 3-(alpha-ethylbenzyl)-4-hydroxycoumarin
20. 3-(alpha-phenylpropyl)-4-hydroxycoumarin
21. 2h-1-benzopyran-2-one, 4-hydroxy-3-(1-phenylpropyl)-
22. 4-hydroxy-3-(1-phenylpropyl)chromen-2-one
23. Phenprocumonum
24. Chembl16694
25. Q08sio485d
26. Chebi:50438
27. Bs-7565
28. Fenprocoumona
29. 3-(.alpha.-ethylbenzyl)-4-hydroxycoumarin
30. Ro-1-4849
31. Coumarin, 3-(.alpha.-ethylbenzyl)-4-hydroxy-
32. 2-hydroxy-3-(1-phenylpropyl)-4h-chromen-4-one
33. Fenprocoumona [inn-spanish]
34. Phenprocoumone [inn-french]
35. Phenprocoumonum [inn-latin]
36. Fenprocumon
37. Liquamar (tn)
38. Hsdb 3248
39. Einecs 207-108-9
40. 3-(1'-phenyl-propyl)-4-oxycoumarin [german]
41. Phenprocoumon (usan/inn)
42. Brn 1291115
43. Unii-q08sio485d
44. Coumarin, 3-(alpha-ethylbenzyl)-4-hydroxy-
45. Phenprocoumon [usan:usp:inn:ban]
46. Phenprocoumon (marcumar)
47. Phenprocoumon [mi]
48. Phenprocoumon [inn]
49. U29342
50. Chembl1465
51. Phenprocoumon [hsdb]
52. Phenprocoumon [usan]
53. Oprea1_002598
54. Schembl43031
55. Bdbm768
56. Phenprocoumon [vandf]
57. Mls006010940
58. Phenprocoumon [mart.]
59. (+/-)-phenprocoumon
60. Phenprocoumon [who-dd]
61. Gtpl6839
62. Schembl1651720
63. Dtxsid5023459
64. Phenprocoumon [orange Book]
65. Bcp01576
66. Hy-a0145
67. S2188
68. 3-(a-ethyl-benzyl)-4-hydroxycoumarin
69. Akos002126557
70. Akos016844146
71. Db00946
72. Ncgc00346442-02
73. 70206-44-9
74. As-77043
75. Smr004703040
76. Cs-0017467
77. Ft-0698417
78. Phenprocoumon 100 Microg/ml In Acetonitrile
79. D05457
80. D82043
81. 4-hydroxy-3-(1-phenylpropyl)-2h-chromen-2-on
82. 435-97-2,53621-47-9 (sodium Salt)
83. Q267896
84. 3-(.alpha.-phenylpropyl)-4-hydroxycoumarin
85. 4-hydroxy-3-(1-phenylpropyl)-2h-chromen-2-one #
86. J-510448
87. Dl-3-(.alpha.-ethylbenzyl)-4-hydroxycoumarin
88. 4-hydroxy-2-oxo-3-(1-phenylpropyl)-2h-chromene
89. Z2186911943
90. 2h-pyran-2-one, 4-hydroxy-3-[[2-(1-methylethyl)phenyl]thio]-6-(3-methylphenyl)-
91. 3-(1-phenylpropyl)-4-hydroxycoumarin; 4-hydroxy-2-oxo-3-(1-phenylpropyl)-2h-chromene
92. 5999-41-7
| Molecular Weight | 280.3 g/mol |
|---|---|
| Molecular Formula | C18H16O3 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 280.109944368 g/mol |
| Monoisotopic Mass | 280.109944368 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 420 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticoagulants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A prothrombopenic anticoagulant with actions and uses similar to those of dicumarol. Its onset of action is 48-72 hr, and its duration of action may be as long as 7 days. ...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 764
Anticoagulants are indicated in the treatment of patients with recent deep vein thrombosis or thrombophlebitis to prevent extension and embolization of the thrombus and to reduce the risk of pulmonary embolism or recurrent thrombus formation. In acute pulmonary embolism or venous thrombosis, anticoagulants are indicated following initial thrombolytic and/or heparin therapy to decrease the risk of extension, recurrence, or death. /Anticoagulants/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 265
Anticoagulants may prevent the formation of mural thrombi in the heart, which may lead to systemic thromboembolism in patients with chronic atrial fibrillation, especially those with rheumatic mitral stenosis, prosthetic heart valves, left atrial enlargement, or cardiomyopathy. In these patients, anticoagulants may decrease the risk of arterial embolism, pulmonary embolism, or subsequent stroke. /Anticoagulants/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 266
For more Therapeutic Uses (Complete) data for PHENPROCOUMON (8 total), please visit the HSDB record page.
Contraindications to oral anticoagulants include pre-existing or coexisting abnormalities of blood coagulation, active bleeding, recent or imminent surgery of the central nervous system or eye, diagnostic or therapeutic procedures with potential for uncontrollable bleeding including lumbar puncture, malignant hypertension, peptic ulceration, pregnancy, threatened abortion, intrauterine device, cerebrovascular hemorrhage, and bacterial endocarditis. Relative contraindications include thrombocytopenia, pericarditis, pericardial effusions, and unreliability of the patient or of patient supervision. /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 308
Most commonly, oral anticoagulant-induced bleeding is minor and consists of bruising, hematuria, epistaxis, conjunctival hemorrhage, minor gastrointestinal bleeding, bleeding from wounds and sites of trauma, and vaginal bleeding. More serious major or fatal bleeding is most commonly gastrointestinal, intracranial, vaginal, retroperitoneal, or related to a wound or site of trauma, although a large variety of other sites of bleeding have been reported. Intracranial bleeding occurs most frequently in patients receiving oral anticoagulants for cerebrovascular disease and most commonly presents as a subdural hematoma, often unassociated with head trauma. Fatal gastrointestinal bleeding is most commonly from a peptic ulcer, although any gastrointestinal lesion may be a potential source of major bleeding. Overall, a bleeding lesion can be identified in about two thirds of cases of oral anticoagulants-related hemorrhage. /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 311
Overall, the bleeding rate of oral anticoagulant therapy is influenced by several factors: the intensity of anticoagulation, either intentionally or inadvertent; the underlying clinical disorder for which anticoagulant therapy is used (with bleeding occurring most frequently in ischemic cerebrovascular disease and venous thromboembolism; and, with bleeding occurring most commonly in the elderly; the presence of adverse drug interactions or comorbid factors such as clinical states potentiating warfarin action, pre-existing hemorrhagic diathesis, malignancy, recent surgery, trauma, or pre-existing potential bleeding sites (e.g., surgical wound, peptic ulcer, recent cerebral hemorrhage, carcinoma of colon); the simultaneous use of aspirin (but not of dipyridamole); and patient reliability (e.g., increased bleeding in alcoholics not due to ethanol-warfarin drug interaction but rather to unreliability of drug intake). /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 310
It is inadvisable to carry out long-term therapy in chronic alcoholic, in individual who may require intensive salicylate therapy, or in cases of malignant hypertension and active tuberculosis. Oral anticoagulant therapy during pregnancy carries significant hemorrhagic risk for fetus. /oral anticoagulants/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1360
For more Drug Warnings (Complete) data for PHENPROCOUMON (21 total), please visit the HSDB record page.
Used for the prevention and treatment of thromboembolic disease including venous thrombosis, thromboembolism, and pulmonary embolism as well as for the prevention of ischemic stroke in patients with atrial fibrillation (AF).
Phenprocoumon, a coumarin anticoagulant, thins the blood by antagonizing vitamin K which is required for the production of clotting factors in the liver. Anticoagulants such as phenprocoumon have no direct effect on an established thrombus, nor do they reverse ischemic tissue damage (damage caused by an inadequate blood supply to an organ or part of the body). However, once a thrombus has occurred, the goal of anticoagulant treatment is to prevent further extension of the formed clot and prevent secondary thromboembolic complications which may result in serious and possibly fatal sequelae.
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
B01AA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AA - Vitamin k antagonists
B01AA04 - Phenprocoumon
Absorption
Bioavailability is close to 100%
Coumarin anticoagulants pass placental barrier. /coumarin anticoagulants/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1359
The disposition of phenprocoumon differed between male and female rats, with a substantially lower apparent volume of distribution and clearance in female rats. Although female rats had a lower sensitivity to the drug, the differences in kinetics caused an apparent equal response to the same doses and a longer duration of effect.
PMID:2901471 Trenk D et al; J Pharm Pharmacol 40: 403-7 (1988)
Samples of urine and feces were collected daily from a normal human volunteer who had received a dose of pseudoracemic phenprocoumon ...containing a tracer dose of 10 microCi of (14C)phenprocoumon... . After 25 days, 96% of the radiolabeled material was recovered (62.8% in urine and 33.3% in feces). ...The urinary excretion pattern was also confirmed in four additional healthy male subjects who received a single oral dose of pseudoracemic phenprocoumon... . All the drug-related materials (both hydroxylated metabolites and parent compound) that were excreted into the urine were extensively conjugated.
PMID:4078699 Toon S et al; J Pharm Sci 74 (10): 1037-40 (1985)
...A study was conducted in 24 healthy volunteers, ages 23-28 yr, who received an oral and an IV dose of phenprocoumon 9 mg at 3 wk intervals. The following mean data were obtained after IV injection: half-life alpha 0.432 hr, half-life beta 128 hr, initial blood level 0.651 ug/ml, volume of distribution 14.41, area under the concn curve (AUC) 121 ugxhr/ml. After oral intake the following mean values were measured: Tmax 2.25 hr, Cmax 1.01 ug/ml, absorption half-life 0.553 hr, initial blood level 0.865 ug/ml, half-life beta 132 hr, AUC 164 ugxhr/ml. A total mean clearance of 20.0 (IV) and 15.1 (oral) ml/hr was calculated within the first 8 hr post dose, while values measured did not differ between 8 and 48 hr post dose. ...
PMID:8032579 Haustein KO, Huller G; Int. J. Clin. Pharmacol. Ther. 32: 192-197 (1994)
Phenprocoumon is stereoselectively metabolized by hepatic microsomal enzymes (cytochrome P-450) to inactive hydroxylated metabolites (predominant route) and by reductases to reduced metabolites. Cytochrome P450 2C9 is the principal form of human liver P-450 responsible for metabolism.
Pooled plasma from patients receiving phenprocoumon anticoagulant therapy was extracted and the following substances were characterized: phenprocoumon, and its 7-hydroxy,4'-hydroxy and 6-hydroxy derivatives; they were identified by HPLC and after methylation by quartz capillary GC-MS using the electron impact and selective ion monitoring modes. This is the first occasion when phenprocoumon metabolites have been identified in plasma; they were unconjugated and in much lower concentrations (43.2 and 2 ng/ml for the 7,4' and 6-hydroxy derivatives, respectively) than the original compound (2000 ng/ml).
de Vries J; Eur J Clin Pharmacol 29(5): p 591-4 (1986)
...The metabolites of /pseudoracemic phenprocoumon/ were identified as the 4'-, 6-, and 7-hydroxy analogues of phenprocoumon. Virtually all of the recovered radioactivity could be accounted for by the parent drug (approximately 40%) and the three metabolites (approximately 60%). The formation of both 4'-(8.1% of administered dose) and 7- (33.4% of administered dose) hydroxyphenprocoumon was highly stereoselective, giving S/R ratios of 2.86 and 1.69, respectively. The formation of 6- (15.5% of administered dose) hydroxyphenprocoumon showed little stereoselectivity (S/R ratio equal to 0.85).
PMID:4078699 Toon S et al; J Pharm Sci 74 (10): 1037-40 (1985)
5-6 days
Phenprocoumon (Marcumar) has a longer plasma half-life /of/ 5 days than warfarin, as well as a somewhat slower onset of action and a longer duration of action (7-14 days).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1531
Phenprocoumon was given orally to 9 patients with biopsy proven liver cirrhosis (dose range 0.12-0.25 mg/kg) and to 7 healthy volunteers (0.23 mg/kg). Concentrations of phenprocoumon were determined using HPLC in plasma and urine samples obtained for 6-7 days after drug administration. The binding of [3H]-phenprocoumon in plasma from all subjects was determined by equilibrium dialysis. Antipyrine plasma concentrations were determined spectrophotometrically following oral administration of antipyrine (1200 mg). The total body clearance of phenprocoumon was higher in the cirrhotic patients (1.64 +/- 0.16 ml/h/kg mean +/- SEM) than in the healthy volunteers (0.90 +/- 0.07 ml/h/kg), however the free drug clearance was not significantly different in the patients (144 +/- 14 ml/h/kg) compared with normal (113 +/- 11 ml/h/kg). In contrast the clearance of antipyrine was much reduced in the cirrhotic group (17.5 +/- 2.9 ml/h/kg) compared with normal (35.6 +/- 3.9 ml/h/kg). The metabolic clearance of phenprocoumon via glucuronidation, is relatively unaffected during cirrhosis compared with antipyrine clearance via oxidation.
Kitteringham N, et al; Eur J Clin Pharmacol 26(1): p 65-70 (1984)
...The following mean data were obtained after IV injection /of phenprocoumon/: half-life alpha 0.432 hr, half-life beta 128 hr... . After oral intake the following mean values were measured: ...absorption half-life 0.553 hr, ...half-life beta 132 hr... .
PMID:8032579 Haustein KO, Huller G; Int. J. Clin. Pharmacol. Ther. 32: 192-197 (1994)
Phenprocoumon inhibits vitamin K reductase, resulting in depletion of the reduced form of vitamin K (vitamin KH2). As vitamin K is a cofactor for the carboxylation of glutamate residues on the N-terminal regions of vitamin K-dependent proteins, this limits the gamma-carboxylation and subsequent activation of the vitamin K-dependent coagulant proteins. The synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S is inhibited. Depression of three of the four vitamin K-dependent coagulation factors (factors II, VII, and X) results in decreased prothrombin levels and a decrease in the amount of thrombin generated and bound to fibrin. This reduces the thrombogenicity of clots.
The oral anticoagulants block the regeneration of reduced vitamin K and thereby induce a state of functional vitamin K deficiency. The mechanism of the inhibition of reductase(s) by the coumarin drugs is not known. There exist reductases that are less sensitive to these drugs but that act only at relatively high concentrations of oxidized vitamin K; this property may explain the observation that administration of sufficient vitamin K can counteract even large doses of oral anticoagulants. /Oral Anticoagulants/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1527
The disposition of a single intravenous bolus dose of 10 mg vitamin K1 and vitamin K1-2,3-epoxide were studied in two healthy subjects without and with 12 hr pretreatment dose of phenprocoumon (0.4 mg/kg). For each compound administered alone the plasma concn-time profile was adequately fitted by a biexponential equation, with an avg terminal half-life of 2.0 and 1.15 hr for the administered vitamin K and its 2,3-epoxide respectively. While vitamin K1 was measurable in plasma following admin of vitamin K1-2,3-epoxide, the epoxide was not detectable following admin of vitamin K1. Following pretreatment with phenprocoumon and after iv admin of vitamin K1, both the avg half-life and area under the plasma concn-time profile of vitamin K1 were marginally reduced to 1.5 hr and 1.76 mg/l/hr respectively, while the plasma concn of vitamin K1-2,3-epoxide was readily measurable and its half-life markedly prolonged to 14.7 hr. Following pretreatment with phenprocoumon and after oral administration of vitamin K1-2,3-epoxide, no vitamin K1 was detectable in plasma and the half-life of the epoxide was 13.8 hr. Based on area considerations the data suggest that either phenprocoumon does more than just inhibit the reduction of vitamin K1-2,3-epoxide to vitamin K1, or that the simple model describing the interconversion between vitamin K1 and its epoxide is inadequate. The same conclusion is drawn from the analysis of comparable data in dogs... .
PMID:6661354 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1428333 Bechtold H, et al; Br J Clin Pharmacol 16 (6): 683-9 (1983)
Both 4-hydroxycoumarin derivatives and indandiones (also known as oral anticoagulants) are antagonists of vitamin K. Their use as rodenticides is based on the inhibition of the vitamin K-dependent step in the synthesis of a number of blood coagulation factors. The vitamin K-dependent proteins ...in the coagulation cascade... are the procoagulant factors II (prothrombin), VII (proconvertin), IX (Christmas factor) and X (Stuart-Prower factor), and the coagulation-inhibiting proteins C and S. All these proteins are synthesized in the liver. Before they are released into the circulation the various precursor proteins undergo substantial (intracellular) post-translational modification. Vitamin K functions as a co-enzyme in one of these modifications, namely the carboxylation at well-defined positions of 10-12 glutamate residues into gamma-carboxyglutamate (Gla). The presence of these Gla residues is essential for the procoagulant activity of the various coagulations factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its oxidation to vitamin K 2,3-epoxide (KO) provides the energy required for the carboxylation reaction. The epoxide is than recycled in two reduction steps mediated by the enzyme KO reductase... . The latter enzyme is the target enzyme for coumarin anticoagulants. Their blocking of the KO reductase leads to a rapid exhaustion of the supply of KH2, and thus to an effective prevention of the formation of Gla residues. This leads to an accumulation of non-carboxylated coagulation factor precursors in the liver. In some cases these precursors are processed further without being carboxylated, and (depending on the species) may appear in the circulation. At that stage the under-carboxylated proteins are designated as descarboxy coagulation factors. Normal coagulation factors circulate in the form of zymogens, which can only participate in the coagulation cascade after being activated by limited proteolytic degradation. Descarboxy coagulation factors have no procoagulant activity (i.e. they cannot be activated) and neither they can be converted into the active zymogens by vitamin K action. Whereas in anticoagulated humans high levels of circulating descarboxy coagulation factors are detectable, these levels are negligible in warfarin-treated rats and mice. /Anticoagulant rodenticides/
WHO; Environ Health Criteria 175: Anticoagulant Rodenticides p.46 (1995)