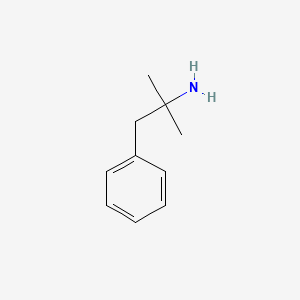

1. Adipex P
2. Adipex-p
3. Adipexp
4. Duromine
5. Hydrochloride, Phentermine
6. Ionamine
7. Phentermine Hydrochloride
1. 122-09-8
2. 2-methyl-1-phenylpropan-2-amine
3. Duromine
4. Ionamin
5. 1,1-dimethyl-2-phenylethylamine
6. Alpha,alpha-dimethylphenethylamine
7. Phenterminum
8. Fentermina
9. Mirapront
10. Lipopill
11. Adipex-p
12. 2-phenyl-tert-butylamine
13. Qsiva
14. Benzeneethanamine, Alpha,alpha-dimethyl-
15. Phentermine Resin
16. Normephentermine
17. Benzeneethanamine, .alpha.,.alpha.-dimethyl-
18. Lonamin
19. Omnibex
20. Linyl
21. .alpha.,.alpha.-dimethylphenethylamine
22. Nsc-759163
23. C045tql4wp
24. Chembl1574
25. Chebi:8080
26. Obermine
27. Phentrol
28. Phentrol 2
29. Phentrol 3
30. Phentrol 4
31. Dea No. 1640
32. 1,1-dimethyl-2-phenyl-ethylamine
33. Phenyl-tert-butylamine
34. Alpha-benzylisopropylamine
35. Phenyl-tertiary-butylamine
36. Fentermina [inn-spanish]
37. Phenterminum [inn-latin]
38. Phentermine [usan:inn:ban]
39. Phentermine Resin Complex
40. 2-amino-2-methyl-1-phenylpropane
41. Rcra Waste Number P046
42. Alpha,alpha-dimethylbenzeneethanamine
43. Alpha,alpha-dimethyl-beta-phenylethylamine
44. Hsdb 3158
45. Sr-05000001805
46. (alpha,alpha)-dimethylphenethylamine
47. Einecs 204-522-1
48. Ethanamine, 1,1-dimethyl-2-phenyl-
49. Phentermine (usan/inn)
50. Rcra Waste No. P046
51. Unii-c045tql4wp
52. Brn 0970319
53. Phenethylamine, Alpha,alpha-dimethyl-
54. Alpha,alpha-dimethylphenethylamine Solution
55. Phentermine [mi]
56. Phentermine [inn]
57. 1-benzyl-iso-propyl Amine
58. Phentermine [hsdb]
59. Phentermine [usan]
60. Ec 204-522-1
61. Phentermine [vandf]
62. Phentermine [mart.]
63. Schembl26615
64. Phentermine [who-dd]
65. Gtpl7269
66. 122-09-8 (free Base)
67. Dtxsid9023461
68. Hms2093b16
69. Pharmakon1600-01505660
70. Qsymia Component Phentermine
71. Phentermine 0.1 Mg/ml In Methanol
72. Phentermine 1.0 Mg/ml In Methanol
73. Zinc8403947
74. .alpha..alpha.dimethylphenethylamine
75. Bdbm50246598
76. Nsc759163
77. Akos004123261
78. Phentermine Component Of Qsymia
79. Ab02355
80. Db00191
81. Nsc 759163
82. Ncgc00263911-02
83. Phenethylamine, .alpha.,.alpha.-dimethyl-
84. Sbi-0206817.p001
85. D1291
86. Ft-0716831
87. C07438
88. D05458
89. Q418157
90. Sr-05000001805-1
91. Sr-05000001805-2
92. Brd-k96319534-001-01-7
93. Phentermine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 149.23 g/mol |
|---|---|
| Molecular Formula | C10H15N |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 149.120449483 g/mol |
| Monoisotopic Mass | 149.120449483 g/mol |
| Topological Polar Surface Area | 26 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 112 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Adipex-p |
| PubMed Health | Phentermine (By mouth) |
| Drug Classes | Appetite Suppressant, Centrally Acting |
| Drug Label | Phentermine hydrochloride USP has the chemical name of ,,-Dimethylphenethylamine hydrochloride. The structural formula is as follows:C10H15N HCl M.W. 185.7Phentermine hydrochloride is a white, odorless, hygroscopic, crystalline powder which i... |
| Active Ingredient | Phentermine hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 37.5mg |
| Market Status | Prescription |
| Company | Teva |
| 2 of 6 | |
|---|---|
| Drug Name | Phentermine resin complex |
| Drug Label | Suprenza is an orally disintegrating tablet (ODT) of phentermine hydrochloride, USP. Phentermine hydrochloride is a sympathomimetic amine anorectic. Its chemical name is ,,-dimethylphenethylamine hydrochloride. The structural formula is as follow... |
| Active Ingredient | Phentermine resin complex |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 15mg base; eq 30mg base |
| Market Status | Prescription |
| Company | Lannett Holdings |
| 3 of 6 | |
|---|---|
| Drug Name | Suprenza |
| Active Ingredient | Phentermine hydrochloride |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 37.5mg; 30mg; 15mg |
| Market Status | Prescription |
| Company | Citius Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Adipex-p |
| PubMed Health | Phentermine (By mouth) |
| Drug Classes | Appetite Suppressant, Centrally Acting |
| Drug Label | Phentermine hydrochloride USP has the chemical name of ,,-Dimethylphenethylamine hydrochloride. The structural formula is as follows:C10H15N HCl M.W. 185.7Phentermine hydrochloride is a white, odorless, hygroscopic, crystalline powder which i... |
| Active Ingredient | Phentermine hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 37.5mg |
| Market Status | Prescription |
| Company | Teva |
| 5 of 6 | |
|---|---|
| Drug Name | Phentermine resin complex |
| Drug Label | Suprenza is an orally disintegrating tablet (ODT) of phentermine hydrochloride, USP. Phentermine hydrochloride is a sympathomimetic amine anorectic. Its chemical name is ,,-dimethylphenethylamine hydrochloride. The structural formula is as follow... |
| Active Ingredient | Phentermine resin complex |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 15mg base; eq 30mg base |
| Market Status | Prescription |
| Company | Lannett Holdings |
| 6 of 6 | |
|---|---|
| Drug Name | Suprenza |
| Active Ingredient | Phentermine hydrochloride |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 37.5mg; 30mg; 15mg |
| Market Status | Prescription |
| Company | Citius Pharms |
Adrenergic Agents; Adrenergic Uptake Inhibitors; Appetite Depressants; Central Nervous System Stimulants; Dopamine Agents; Dopamine Uptake Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... Phentermine /is/ indicated in the short-term (a few weeks) treatment of exogenous obesity in conjunction with a regimen of weight reduction based on caloric restriction, exercise, and behavior modification in patients with a body mass index of > or = 30 kg of body weight per height in meters squared (kg/sq m) or in patients with a body mass index of > or = 27 kg/sq m in the presence of risk factors such as hypertensin, diabetes, or hyperlipidemia. /Included in US product labeling /
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 438
Anorexic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1304
... /Phentermine hydrochloride & phentermine resin/ were developed in hope that they would produce greater anorexiant effect with fewer untoward reactions than amphetamines. In spite of minor differences in their actions & untoward effects ... /neither/ has been found to be more effective than dextroamphetamine. /Phentermine hydrochloride and Phentermine resin/
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 487
Pulmonary hypertension has been reported in patients receiving phentermine in combination with dexfenfluramine (no longer commercially available in the US), fenfluramine (no longer commercially available in the US), and in those with a history of receiving at least one other anorexigenic agent; however, the possibility of an association between pulmonary hypertension and the use of phentermine as monotherapy cannot be ruled out. Primary pulmonary hypertension is a rare, frequently fatal pulmonary disease. The initial symptom of pulmonary hypertension generally is dyspnea. Other initial manifestations include angina pectoris, syncope, or edema of the lower extremities. Phentermine should be discontinued in any patient who develops new, unexplained symptoms of dyspnea, angina, syncope, or edema of the lower extremities. Such patients should be evaluated for the possible presence of pulmonary hypertension. In addition, patients receiving phentermine should be advised to report immediately any deterioration in exercise tolerance
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2384
Valvular Heart Disease: Serious regurgitant cardiac valvular disease, primarily affecting the mitral, aortic and/or tricuspid valves, has been reported in otherwise healthy persons who had taken a combination of phentermine with fenfluramine or dexfenfluramine for weight loss. The etiology of these valvulopathies has not been established and their course in individuals after the drugs are stopped is not known. The possibility of an association between valvular heart disease and the use of phentermine alone cannot be ruled out; there have been rare cases of valvular heart disease in patients who reportedly have taken phentermine alone.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1324
Phentermine should not be used in combination with selective serotonin-reuptake inhibitor antidepressants /SSRI/ (e.g., fluoxetine, fluvoxamine, paroxetine, sertraline) or MAO inhibitors, since severe adverse reactions may occur. In addition, one manufacturer of phendimetrazine tartrate (Plegine) states that phendimetrazine should not be used in combination with other anorexigenic agents (e.g., phentermine) since valvulopathy and primary pulmonary hypertension have been reported in patients receiving phendimetrazine who had received at least one other anorexigenic agent.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2384
Habituation or addiction has been reported with similar drugs and the possibility of its occurrence should be considered with phentermine.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2384
For more Drug Warnings (Complete) data for PHENTERMINE (15 total), please visit the HSDB record page.
Phentermine is indicated, alone or in combination with topiramate, as a short-term adjunct, not pass a few weeks, in a regimen of weight reduction based on exercise, behavioral modifications and caloric restriction in the management of exogenous obesity for patients with an initial body mass index (BMI) greater than 30 kg/m2 or greater than 27 kg/m2 in presence of other risk factors such as controller hypertension, diabetes or hyperlipidemia. Exogenous obesity is considered when the overweight is caused by consuming more food than the person activity level warrants. This condition commonly causes an increase in fat storage. It is an epidemic condition in the United States where over two-thirds of adults are overweight or obese and one in three Americans is obese. In the world, the incidence of obesity has nearly doubled.
FDA Label
It is reported that the main mechanism of action of phentermine is the generation of appetite suppression, maybe due to the increase in leptin, but it is considered that other mechanisms should be involved. Some reports have indicated that the weight loss effect is mainly due to the increase in resting energy expenditure. In clinical studies where phentermine was used as a monotherapy and as combination therapy, this drug has shown an average weight loss of 3.6 kg when compared with the placebo in 2-24 weeks. Patients treated with phentermine also showed increased maintenance of the weight after treatment discontinuation. As well, even though it is a derivative of the amphetamines, it has not been registered to produce any of the effects of amphetamine such as central nervous system stimulation, elevation of blood pressure, tachyphylaxis or QTc prolongation.
Appetite Depressants
Agents that are used to suppress appetite. (See all compounds classified as Appetite Depressants.)
Adrenergic Agents
Drugs that act on adrenergic receptors or affect the life cycle of adrenergic transmitters. Included here are adrenergic agonists and antagonists and agents that affect the synthesis, storage, uptake, metabolism, or release of adrenergic transmitters. (See all compounds classified as Adrenergic Agents.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
A - Alimentary tract and metabolism
A08 - Antiobesity preparations, excl. diet products
A08A - Antiobesity preparations, excl. diet products
A08AA - Centrally acting antiobesity products
A08AA01 - Phentermine
Absorption
Phentermine shows a dose-dependent pharmacokinetic profile. After oral administration of a dose of 15 mg, the maximal concentration was achieved after 6 hours and its bioavailability was not affected by the consumption of high-fat meals. The reported plasma concentration at steady-state is of around 200 ng/ml as observed in clinical trials.
Route of Elimination
Phentermine is excreted mainly in the urine from which about 70-80% of the administered dose can be found as the unchanged drug.
Volume of Distribution
The reported volume of distribution for phentermine is reported to be of 5 L/kg.
Clearance
The reported clearance when administered orally is 8.79 L/h as observed in pharmacokinetic population studies.
Absorbed readily after oral administration. Distributed widely throughout body.
Cowl, C.T. Physician's Handbook 10th edition. Lippincott Williams & Wilkins, Philadelphia, PA. 2003, p. 973
The volume of distribution is 3 to 4 L/kg ... A majority of the drug is excreted unchanged in the urine. An average of 48% of the dose is excreted in the urine in 24 hours, which increases to 84% in acidic urine.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 877
Elimination is primarily renal; excretion is increased by acidifying the urine.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 438
N-hydroxyphentermine is rapidly reduced back to phentermine. This pathway recycles parent drug, which then has a longer duration of action.
Jenden DJ et al; In Central Mechanisms Of Anorectic Drugs; Garattini S, Samanin R, eds, p. 165-77 (1978)
Phentermine undergoes minimal p-hydroxylation, N-oxidation and N-hydroxylation followed by conjugation. The total proportion of the drug that goes under metabolism only represents about 6% of the administered dose where about 5% is represented by the N-oxidized and N-Hydroxylated metabolites.
Phentermine is not significantly biotransformed; 70 to 80 % of a given dose is excreted unchanged in the urine.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 438
There are two primary pathways of oxidative metabolism: n-hydroxylation to n-hydroxyphentermine & p-hydroxylation to para-hydroxyphentermine. N-hydroxyphentermine is metabolized further to alpha-nitrophentermine. In rats the dominant pathway is p-hydroxylation to give para-hydroxyphentermine, accounting for more than 60% of administered dose. P-hydroxylation reactions in rat liver microsomes indicated that phentermine metabolism was inhibited by skf525a & iprindol, and was not inducible with phenobarbital or 3-methylcholanthrene, suggesting a system different from aromatic c-hydroxylation. The n-hydroxylation reaction for rabbit & guinea pig (two-enzyme system) & rat (single enzyme system) is the reduced form of nicotinamide-adenine dinucleotide phosphate-dependent, inhibited by carbon monoxide & induced by phenobarbital, suggesting involvement of cytochrome p450 system.
Jenden DJ et al; In Central Mechanisms Of Anorectic Drugs; Garattini S, Samanin R, eds, p. 165-77 (1978)
Although no quantitative data are available, urinary metabolites found in man are p-hydroxyphentermine and n-hydroxyphentermine. N-hydroxy metabolite can be reduced in vivo but conversion to this metabolite appears to be inefficient means of elimination.
Jenden DJ et al; In Central Mechanisms Of Anorectic Drugs; Garattini S, Samanin R, eds, p. 165-77 (1978)
The role of the cytochrome p450 system in the oxidation of phentermine to N-hydroxyphentermine by liver microsomal prepn was studied in a reconstituted system which consisted of cytochrome p450 and the reduced form of nicotinamide-adenine dinucleotide phosphate cytochrome p450 reductase purified from liver microsomes of phenobarbital-induced rabbits. In this system, phentermine was oxidized to N-hydroxyphentermine. The reaction was the reduced form of nicotinamide-adenine dinucleotide phosphate-dependent and required the presence of both the cytochrome p450 and reductase preparations.
PMID:6815477 Duncan JD, Cho AK; Mol Pharmacol 22 (2): 235-36 (1982)
The mean terminal half-life of phentermine is reported to be of approximately 20 hours. In conditions where there is acidic urine (pH <5), the elimination half-life is of 7-8 hours.
... Elimination half-life is approximately 7-8 hours in acid urine (pH5), compared with 19-24 hours under normal conditions.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 631
S(+)-phentermine was eliminated much more rapidly in rat (k= 1.32/hr) after iv administration than in man (k= 0.029/hr) after oral dose. Half-lives were 25-35 min in rat & 12-24 hr in man.
Jenden DJ et al; In Central Mechanisms Of Anorectic Drugs; Garattini S, Samanin R, eds, p. 165-77 (1978)
Phentermine is an indirect-acting sympathomimetic agent that acts by releasing noradrenaline from the presynaptic vesicles in the lateral hypothalamus. This increase in noradrenaline concentration in the synaptic cleft results in the stimulation of beta2-adrenergic receptors. Phentermine is classified as an indirect sympathomimetic due to the increase in the level of norepinephrine, dopamine and its indirect effect towards serotonin. Some reports have indicated that phentermine inhibits the neuropeptide Y which is a principal signaling pathway for the induction of hunger. This combined effect produces a continuous flight-or-fight response in the body which reduces the hunger signal as this state is on the immediate need for energy. Lastly, some reports have indicated that phentermine is a weak inhibitor of monoamine oxidase but this mechanism does not tend to produce a clinically significant response.
Although the mechanism of action of the sympathomimetic appetite suppressants in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Amphetamine and related sympathomimetic medications ... are thought to stimulate the release of norepinephrine and/or dopamine from storage sites in nerve terminals in the lateral hypothalamic feeding center, thereby producing a decrease in appetite. ... It has not been established that the actions of these medications in treating obesity is primarily suppression of appetite; other CNS actions and/or metabolic effects, such as decreased gastric acid secretion or increased energy expenditure, may be involved.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 439