
1. Endorphenyl
2. L-isomer Phenylalanine
3. Phenylalanine, L Isomer
4. Phenylalanine, L-isomer
1. 63-91-2
2. 3-phenyl-l-alanine
3. (2s)-2-amino-3-phenylpropanoic Acid
4. (s)-2-amino-3-phenylpropanoic Acid
5. (s)-phenylalanine
6. (s)-2-amino-3-phenylpropionic Acid
7. Beta-phenyl-l-alanine
8. (l)-phenylalanine
9. 3-phenylalanine
10. H-phe-oh
11. Antibiotic Fn 1636
12. L-alanine, Phenyl-
13. Alanine, 3-phenyl-
14. L-antibiotic Fn 1636
15. (s)-alpha-amino-beta-phenylpropionic Acid
16. L-alanine, 3-phenyl-
17. Phenylalanine (van)
18. Fenilalanina [spanish]
19. Phenylalaninum [latin]
20. (s)-alpha-aminohydrocinnamic Acid
21. Alanine, Phenyl-, L-
22. Fema No. 3585
23. (s)-(-)-phenylalanine
24. Beta-phenylalnine, (-)-
25. (s)-alpha-amino-benzenepropanoic Acid
26. Phenylalanine, L-
27. Hydrocinnamic Acid, Alpha-amino-
28. 2-amino-3-phenylpropionic Acid, L-
29. Phenylalanine [usan:inn:jan]
30. Hsdb 1825
31. Alpha-aminohydrocinnamic Acid, L-
32. Alpha-amino-beta-phenylpropionic Acid, L-
33. Benzenepropanoic Acid, Alpha-amino-, (s)-
34. Fenilalanina
35. Phe
36. Alpha-aminohydrocinnamic Acid
37. Endophenyl
38. Nsc 79477
39. L-phe
40. Laevo-phenyl Alanine
41. 67675-33-6
42. L-phenylalinine
43. Chembl301523
44. Chebi:17295
45. 47e5o17y3r
46. Nsc-79477
47. Phenylalaninum
48. Mfcd00064227
49. Phenylalamine
50. Phenylalanine (l-phenylalanine)
51. Alanine, Phenyl-
52. .beta.-phenylalanine
53. Phenyl-.alpha.-alanine
54. L-.beta.-phenylalanine
55. .beta.-phenyl-l-alanine
56. (-)-.beta.-phenylalanine
57. Einecs 200-568-1
58. .alpha.-aminohydrocinnamic Acid
59. .beta.-phenyl-.alpha.-alanine
60. Phenylalanine (usp/inn)
61. L-phenylaniline
62. Hydrocinnamic Acid, .alpha.-amino-
63. Unii-47e5o17y3r
64. Ccris 4254
65. .beta.-phenyl-.alpha.-alanine, L-
66. L-phenyl Alanine
67. Pheoh
68. 1usi
69. Nci9959
70. (-)-phenylalanine
71. .alpha.-amino-.beta.-phenylpropionic Acid
72. Racemic Phenylalanine
73. Phenylalanine [usan:usp:inn:jan]
74. 1f2p
75. Alpha-aminohydrocinnamate
76. (-)-beta-phenylalanine
77. L-phenylalanine, 99%
78. Beta-phenyl-alpha-alanine
79. L-phenylalanine (jp17)
80. Phenylalanine [ii]
81. Phenylalanine [mi]
82. Bmse000045
83. Bmse000900
84. Bmse000921
85. Bmse001016
86. Phenylalanine [inn]
87. Schembl8119
88. Ncistruc1_000204
89. Ncistruc2_000248
90. H-phe-2-chlorotrityl Resin
91. Phenylalanine [hsdb]
92. Phenylalanine [inci]
93. Phenylalanine [usan]
94. L-(-)-phenylalanine
95. Phenylalanine [vandf]
96. (s)-alpha-aminohydrocinnamate
97. L-phenylalanine (h-phe-oh)
98. L-phenylalanine [fcc]
99. L-phenylalanine [jan]
100. Phenylalanine [mart.]
101. L-2-amino-3-phenylpropionate
102. L-phenylalanine [fhfi]
103. L-phenylalanine, 99%, Fcc
104. Phenylalanine [who-dd]
105. Gtpl3313
106. (s)-alpha-aminobenzenepropanoate
107. 3-amino-4-phenyl-butanoic Acid
108. Dtxsid4040763
109. (s)-2-amino-3-phenylpropanoate
110. Bdbm18073
111. (s)-2-amino-3-phenylpropionate
112. 1f9436b3-8b0d-4ac6-a004-4249b0bda436
113. L-phenylalanine [usp-rs]
114. L-phenylalanine Non-animal Source
115. (s)-alpha-amino-benzenepropanoate
116. L-2-amino-3-phenylpropionic Acid
117. Zinc105196
118. Hy-n0215
119. L-2-amino-3-phenyl-propionic Acid
120. Phenylalanine [ep Monograph]
121. (s)-alpha-aminobenzenepropanoic Acid
122. Ac8117
123. Ccg-37572
124. Ncgc00013103
125. Phenylalanine [usp Monograph]
126. L-[2,3,4,5,6-3h]phenylalanine
127. (s)-alpha-amino-beta-phenylpropionate
128. Akos010373257
129. Akos015853585
130. (s)-.alpha.-aminobenzenepropanoic Acid
131. Db00120
132. L-phenylalanine, Vetec(tm), 98.5%
133. L-phenylalanine, Reagent Grade, >=98%
134. Leucine Impurity C [ep Impurity]
135. Ncgc00013103-02
136. Ncgc00013103-03
137. Ncgc00013103-04
138. Ncgc00013103-05
139. Ncgc00095047-01
140. Ncgc00095047-02
141. Ncgc00095047-03
142. Ncgc00095047-04
143. Ac-22417
144. As-14129
145. Bp-20538
146. Tyrosine Impurity A [ep Impurity]
147. Db-029978
148. L-phenylalanine, 99%, Natural, Fcc, Fg
149. Am20060774
150. Nateglinide Impurity D [ep Impurity]
151. P0134
152. Benzenepropanoic Acid, .alpha.-amino-, (s)-
153. L-phenylalanine, Bioultra, >=99.0% (nt)
154. 63p912
155. A20654
156. C00079
157. D00021
158. M02961
159. L-phenylalanine, Saj Special Grade, >=99.0%
160. Q170545
161. L-.alpha.-amino-.beta.-phenylpropionic Acid
162. L-phenylalanine, Vetec(tm) Reagent Grade, >=98%
163. Q-201326
164. L-phenylalanine, Cell Culture Reagent (h-l-phe-oh)
165. Lysine Hydrochloride Impurity B [ep Impurity]
166. F0001-2360
167. Z1258578341
168. L-phenylalanine, Certified Reference Material, Tracecert(r)
169. Phenylalanine, European Pharmacopoeia (ep) Reference Standard
170. 3-(2-aminoethyl)-1,3-thiazolidine-2,4-dionehydrochloride
171. L-phenylalanine, United States Pharmacopeia (usp) Reference Standard
172. L(-)-phenylalanine; Beta-phenylalanine;dl-2-amino-3-phenylpropanoic Acid;
173. L-phenylalanine, Analytical Standard, For Nitrogen Determination According To Kjeldahl Method
174. L-phenylalanine, Pharmaceutical Secondary Standard; Certified Reference Material
175. L-phenylalanine, From Non-animal Source, Meets Ep, Jp, Usp Testing Specifications, Suitable For Cell Culture, 98.5-101.0%
176. L-phenylalanine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 165.19 g/mol |
|---|---|
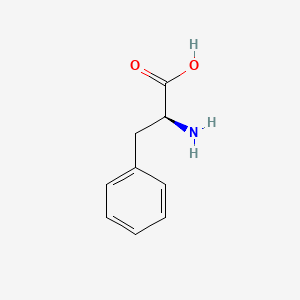
| Molecular Formula | C9H11NO2 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.078978594 g/mol |
| Monoisotopic Mass | 165.078978594 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 153 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
An essential aromatic amino acid that is a precursor of MELANIN; DOPAMINE; noradrenalin (NOREPINEPHRINE), and THYROXINE.
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/EXPTL TREATMENT/ There is no totally effective treatment for vitiligo (localised hypopigmentation). Oral or topical photochemotherapy with psoralens is generally considered to be the best available treatment, but experimental therapy includes UVA phototherapy with phenylalanine. Use of phenylalanine in oral doses of up to 100 mg/kg with UVA/sunlight led to beneficial results in more than 90% of 200 patients with vitiligo. Greatest benefit was noted in early disease, but prolonged use still induced repigmentation in long-standing cases. Repigmentation occurred mainly in areas rich in follicles. Such therapy is contra-indicated in phenylketonuria and in pregnancy. Similarly a further open study reported responses in 94 of 149 patients receiving 50 to 100 mg/kg daily of phenylalanine plus twice weekly UVA treatment. However, only 22% of responders had repigmentation in more than 60% of the affected area. Higher doses did not seem to be more effective than 50 mg/kg daily. Another group reported on 6 years of experience of treatment of vitiligo using 50 or 100 mg/kg daily of phenylalanine, with application of 10% phenylalanine gel and daily sun exposure. Although not ideal, they considered the treatment useful, especially for its ability to rapidly repigment the face. The same group performed an open study, adding topical 0.025% clobetasol propionate, and ultraviolet exposure during autumn and winter; 65.5% of patients achieved 100% repigmentation on the face.
Sweetman SC (ed), Martindale: The Complete Drug Reference. London: Pharmaceutical Press (2009), p.1960.
/Experimental Therapy/ L-Phenylalanine (Phe), is a potent releaser of the satiety hormone, cholecystokinin (CCK) and previous studies, conducted primarily in men, show that ingestion of Phe reduces energy intake. The objective of the current study was to test the effects of Phe on energy intake in overweight and obese women. Subjects (n =3 2) received three treatments (high-dose (10 g Phe), low-dose (5 g Phe and 5 g glucose) or control (10 g glucose)) 20 min before an ad libitum lunch and dinner meal in a within-subjects', counterbalanced, double-blind study. No effect of Phe was found; however, interactions with dietary restraint status were detected in post-hoc analyses.
PMID:18342398 Pohle-Krauza RJ, et al; Appetite 51 (1): 111-9 (2008).
/Experimental Therapy/ L-phenylalanine in combination with 0.025% clobetasol propionate and sunlight during sunny months or UVA lamps in winter, appears to improve evolutive vitiligo without side effects, and therefore is especially recommended on the face or for children.
PMID:12847735 Camacho F, Mazuecos J; J Drugs Dermatol 1 (2): 127-31 (2002).
For more Therapeutic Uses (Complete) data for (L)-Phenylalanine (7 total), please visit the HSDB record page.
Overweight and obese women (n = 32) received three treatments (high-dose (10 g Phe), low-dose (5 g Phe and 5 g glucose) or control (10 g glucose)) 20 min before an ad libitum lunch and dinner meal. High-dose Phe increased ratings of nausea.
PMID:18342398 Pohle-Krauza RJ, et al; Appetite 51 (1): 111-9 (2008).
L-phenylalanine may be helpful in some with depression. It may also be useful in the treatment of vitiligo. There is some evidence that L-phenylalanine may exacerbate tardive dyskinesia in some schizophrenic patients and in some who have used neuroleptic drugs.
Used by the brain to produce Norepinephrine, a chemical that transmits signals between nerve cells and the brain; keeps you awake and alert; reduces hunger pains; functions as an antidepressant and helps improve memory.
Absorption
Absorbed from the small intestine by a sodium dependent active transport process.
... It diffuses across placental membrane reaching higher fetal than maternal levels. In rhesus monkey when serum maternal levels are 1-2 mg/100 mL near full term there is an approx 1.5:1 diffusion rate, but when maternal levels ... high (25 mg/100 mL) fetal serum ... reach 45 mg/100 mL to detriment of fetus. /Phenylalanine/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 448
Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 596, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Table: Comparison of the Pool Sizes of Free and Protein-Bound Amino Acids in Rat Muscle [Table#3670]
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 597, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
[Table#3670]
After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bondspecific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 599, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
For more Absorption, Distribution and Excretion (Complete) data for (L)-Phenylalanine (13 total), please visit the HSDB record page.
Hepatic. L-phenylalanine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body, where it undergoes metabolic reactions similar to those that take place in the liver.
Pathways of amino acid metabolism- L-phenylalanine; product of oxidative deamination or transamination: phenylpyruvic acid. Product of decarboxylation: phenylethylamine. Phenylalanine to tyrosine.
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 831
L-Phenylalanine yields in man: N-acetyl-L-phenylalanine; benzoic acid; probably in man, 2,5-dihydroxy-L-phenylalanine. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. 15
L-Phenylalanine yields in man: phenethylamine; phenylpyruvic acid; L-tyrosine. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. 16
L-Phenylalanine yields L-m-tyrosine in rat. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. 16
For more Metabolism/Metabolites (Complete) data for (L)-Phenylalanine (12 total), please visit the HSDB record page.
The supposed antidepressant effects of L-phenylalanine may be due to its role as a precursor in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects.
The mechanism of L-phenylalanine's possible antivitiligo activity is not well understood. It is thought that L-phenylalanine may stimulate the production of melanin in the affected skin
Amino acids are selected for protein synthesis by binding with transfer RNA (tRNA) in the cell cytoplasm. The information on the amino acid sequence of each individual protein is contained in the sequence of nucleotides in the messenger RNA (mRNA) molecules, which are synthesized in the nucleus from regions of DNA by the process of transcription. The mRNA molecules then interact with various tRNA molecules attached to specific amino acids in the cytoplasm to synthesize the specific protein by linking together individual amino acids; this process, known as translation, is regulated by amino acids (e.g., leucine), and hormones. Which specific proteins are expressed in any particular cell and the relative rates at which the different cellular proteins are synthesized, are determined by the relative abundances of the different mRNAs and the availability of specific tRNA-amino acid combinations, and hence by the rate of transcription and the stability of the messages. From a nutritional and metabolic point of view, it is important to recognize that protein synthesis is a continuing process that takes place in most cells of the body. In a steady state, when neither net growth nor protein loss is occurring, protein synthesis is balanced by an equal amount of protein degradation. The major consequence of inadequate protein intakes, or diets low or lacking in specific indispensable amino acids relative to other amino acids (often termed limiting amino acids), is a shift in this balance so that rates of synthesis of some body proteins decrease while protein degradation continues, thus providing an endogenous source of those amino acids most in need. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 601-602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The mechanism of intracellular protein degradation, by which protein is hydrolyzed to free amino acids, is more complex and is not as well characterized at the mechanistic level as that of synthesis. A wide variety of different enzymes that are capable of splitting peptide bonds are present in cells. However, the bulk of cellular proteolysis seems to be shared between two multienzyme systems: the lysosomal and proteasomal systems. The lysosome is a membrane-enclosed vesicle inside the cell that contains a variety of proteolytic enzymes and operates mostly at acid pH. Volumes of the cytoplasm are engulfed (autophagy) and are then subjected to the action of the protease enzymes at high concentration. This system is thought to be relatively unselective in most cases, although it can also degrade specific intracellular proteins. The system is highly regulated by hormones such as insulin and glucocorticoids, and by amino acids. The second system is the ATP-dependent ubiquitin-proteasome system, which is present in the cytoplasm. The first step is to join molecules of ubiquitin, a basic 76-amino acid peptide, to lysine residues in the target protein. Several enzymes are involved in this process, which selectively targets proteins for degradation by a second component, the proteasome. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html