
1. A-1325912.0
2. Abt-530
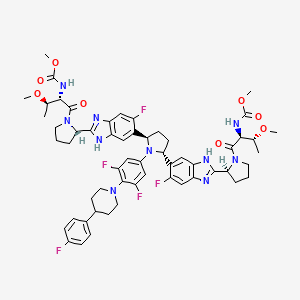
3. Dimethyl N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)((2s)-pyrrolidine-2,1-diyl)((2s,3r)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
1. 1353900-92-1
2. Abt-530
3. Pibrentasvir [usan]
4. Abt 530
5. A-1325912.0
6. Abt530
7. 2wu922tk3l
8. 1353900-92-1 (free)
9. Dimethyl N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)((2s)-pyrrolidine-2,1-diyl)((2s,3r)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
10. Dimethyl ((2s,2's,3r,3'r)-((2s,2's)-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis(6-fluoro-1h-benzo[d]imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methoxy-1-oxobutane-1,2-diyl))dicarbamate
11. Methyl N-[(2s,3r)-1-[(2s)-2-[6-[(2r,5r)-1-[3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl]-5-[6-fluoro-2-[(2s)-1-[(2s,3r)-3-methoxy-2-(methoxycarbonylamino)butanoyl]pyrrolidin-2-yl]-3h-benzimidazol-5-yl]pyrrolidin-2-yl]-5-fluoro-1h-benzimidazol-2-yl]pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl]carbamate
12. Unii-2wu922tk3l
13. Abt-530;pibrentasvir
14. Pibrentasvir [mi]
15. Pibrentasvir(abt-530)
16. Pibrentasvir [inn]
17. Pibrentasvir [jan]
18. Pibrentasvir (abt-530)
19. Pibrentasvir [who-dd]
20. Pibrentasvir (jan/usan/inn)
21. Schembl2756579
22. Chembl3545123
23. Schembl17639956
24. Gtpl11268
25. Ex-a865
26. Dtxsid601027946
27. Pibrentasvir [orange Book]
28. C57h65f5n10o8
29. Bdbm50453100
30. Mavyret Component Pibrentasvir
31. S9641
32. Cs-8135
33. Db13878
34. Ac-33418
35. Bs-15250
36. J3.646.121g
37. D10816
38. J-690144
39. Q47495788
40. A 1325912.0
41. Carbamic Acid, N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)-1-piperidinyl)phenyl)-2,5-pyrrolidinediyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)-(2s)-2,1-pyrrolidinediyl((1s)-1-((1r)-1-methoxyethyl)-2-oxo-2,1-ethanediyl)))bis-, C,c'-dimethyl Ester
42. Methyl ((2s,3r)-1-((2s)-2-(5-((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)-5-(6-fluoro-2-((2s)-1-(n-(methoxycarbonyl)-o-methyl-l-threonyl)pyrrolidin-2-yl)-1h-benzimidazol-5-yl)pyrrolidin-2-yl)-6-fluoro-1h-benzimidazol-2-yl)pyrrolidin-1-yl)-3-methoxy-1-oxobutan-2-yl)carbamate
| Molecular Weight | 1113.2 g/mol |
|---|---|
| Molecular Formula | C57H65F5N10O8 |
| XLogP3 | 7.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1112.49069988 g/mol |
| Monoisotopic Mass | 1112.49069988 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 80 |
| Formal Charge | 0 |
| Complexity | 2000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both.
FDA Label
Pibrentasvir is a pan-genotypic . According to HCV replicon assays, pibrentasvir has EC50 values ranging from 0.08-4.6 nM agaisnt laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4d, 5a, and 6a, or EC50 values of 0.5-4.3 pM against laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4b, 4d, 5a, 6a, 6e and 6p. It is active against common resistance-conferring substitutions in HCV genotypes 1 to 6 that confers resistance and decreased therapeutic response from other NS5A inhibitors, inluding positions 24, 28, 30, 31, 58, 92, or 93 in NS5A. In a QT study, pibrentasvir is not shown to prolong the QTc interval.
Absorption
In healthy subjects, the time it takes to reach the peak plasma concentration (Tmax) is approximately 5 hours. The mean peak plasma concentration (Cmax) is 110ng/mL in non-cirrhotic HCV-infected subjects. Relative to fasting conditions, the consumption of meals increases the absorption of pibrentasvir by 40-53%.
Route of Elimination
The predominant route of elimination of the drug is biliary-fecal, where 96.6% of administered drug is excreted in feces and 0% of the drug is excreted in the urine.
Pibrentasvir is not metabolized.
The elimination half life (t1/2) is approximately 13 hours.
NS5A is a phosphoprotein that plays an essential role in replication, assembly and maturation of infectious viral proteins. The basal phosphorylated form of NS5A, which is maintained by C-terminal serine cluster, is key in ensuring its interaction with the viral capsid protein, or the core protein. By blocking this interaction, pibrentasvir inhibits the assembly of proteins and production of mature HCV particles. NS5A also interacts with viral and cellular proteins to form the HCV replicase complex, and supports the RNA replication of HCV.