

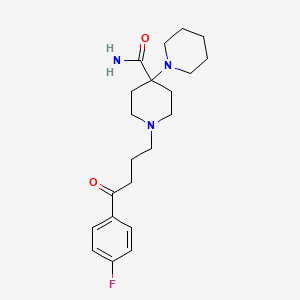

1. 1'-(3-(4-fluorobenzoyl)propyl)-(1,4'-bipiperidine) -4'-carboxamide
2. Dipiperon
3. Pipamperon-neuraxpharm
4. Pipamperone Dihydrochloride
5. R 3345
1. Floropipamide
2. 1893-33-0
3. Dipiperon
4. Dipiperone
5. Dipiperal
6. Piperonyl
7. Piperonil
8. R 3345
9. Pipamperona
10. Pipamperonum
11. Pipamperone Hydrochloride
12. Mcn-jr-3345
13. Pipaneperone
14. Pipamperone Free Base
15. Pipamperone Dihydrochloride Approx. 99
16. Nsc-759178
17. 1'-(3-(p-fluorobenzoyl)propyl)-(1,4'-bipiperidine)-4'-carboxamide
18. (1,4'-bipiperidine)-4'-carboxamide, 1'-(3-(p-fluorobenzoyl)propyl)-
19. Chebi:78549
20. 1893-33-0 (free Base)
21. 4'-fluoro-4-(4-n-piperidino-4-carbamidopiperidino)butyrophenone
22. P-fluoro-gamma-(4-piperidino-4-carbamoylpiperidino)butyrophenone
23. R-3345
24. Carpiperone
25. Fluoropipamide
26. [1,4'-bipiperidine]-4'-carboxamide, 1'-[4-(4-fluorophenyl)-4-oxobutyl]-
27. 1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidin-1-ylpiperidine-4-carboxamide
28. Floropipamide; Mcn-jr 3345; R 3345
29. 5402501f0w
30. 1'-[4-(4-fluorophenyl)-4-oxobutyl]-1,4'-bipiperidine-4'-carboxamide
31. (1,4'-bipiperidine)-4'-carboxamide, 1'-(4-(4-fluorophenyl)-4-oxobutyl)-
32. Pipamperon
33. 1'-(4-(4-fluorophenyl)-4-oxobutyl)-[1,4'-bipiperidine]-4'-carboxamide
34. Pipamperonum [inn-latin]
35. Pipamperona [inn-spanish]
36. Pipamperone [usan:inn:ban]
37. Ccris 9071
38. Ncgc00165864-02
39. Brn 0496532
40. 1'-(4-(4-fluorophenyl)-4-oxobutyl)-1,4'-bipiperidine-4'-carboxamide
41. 1'-[4-(4-fluorophenyl)-4-oxobutyl]-[1,4'-bipiperidine]-4'-carboxamide
42. Unii-5402501f0w
43. Propitan (salt/mix)
44. Isonipacotamide, 1-(3-(p-fluorobenzoyl)propyl)-4-piperidino-
45. Pipamperone [mi]
46. Pipamperone [inn]
47. Pipamperone (usan/inn)
48. Pipamperone [usan]
49. Gtpl92
50. Schembl2412
51. Pipamperone [mart.]
52. Pipamperone [who-dd]
53. 5-22-13-00536 (beilstein Handbook Reference)
54. Mcn-jr 3345
55. Chembl440294
56. Dtxsid8048369
57. Bdbm81483
58. Hms3264g14
59. Pharmakon1600-01505690
60. Nsc_4830
61. 1'-(3-(p-fluorobenzoyl)propyl)(1,4'-bipiperidine)-4'-carboxamide
62. Mfcd00242979
63. Nsc759178
64. Pdsp1_001560
65. Pdsp2_001544
66. Zinc21297287
67. Ccg-213514
68. Db09286
69. Nsc 759178
70. Ncgc00165864-01
71. Ac-32938
72. Pipamperonedihydrochlorideapprox.99
73. Cas_1893-33-0
74. Sbi-0206899.p001
75. Hy-100703
76. Cs-0020023
77. P2315
78. D02622
79. Ab01563106_01
80. A929272
81. L000727
82. Q415118
83. Sr-05000001930
84. Sr-05000001930-1
85. Brd-k26801045-001-01-1
86. Isonipecotamide, 1-(3-(p-fluorobenzoyl)propyl)-4-piperidino-
87. P-fluoro-.gamma.-(4-piperidino-4-carbamoylpiperidino)butyrophenone
88. 1,4'-bipiperidine)-4'-carboxamide, 1'-(4-(4-fluorophenyl)-4-oxobutyl)-
89. 1-(4-fluorophenyl)-4-(4-piperidino-4-carbamoylpiperidino)-1-butanone
| Molecular Weight | 375.5 g/mol |
|---|---|
| Molecular Formula | C21H30FN3O2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 375.23220537 g/mol |
| Monoisotopic Mass | 375.23220537 g/mol |
| Topological Polar Surface Area | 66.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 506 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of chronic psychoses and states of aggressiveness of various origins.
Pipamperone is an antipsychotic medication that has sedative effects, which may be beneficial in the management of agitation and disordered sleep. Pipamperone, showing antidopaminergic and anti-serotonergic properties, has been noted for its anti- agitation effects and for its ability to normalize sleep rhythms in psychiatric patients. One study showed that pipamperone increased the expression of D4 (dopaminergic) receptors, explaining its helpfulness in decreasing positive psychotic symptoms, such as delusions and hallucinations.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
N05AD05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AD - Butyrophenone derivatives
N05AD05 - Pipamperone
Route of Elimination
Mainly via the kidneys
Pipamperone is metabolised in the liver.
17-26h
Pipamperone binds mainly to 5-HT2A receptors, with a nearly equal affinity to D4 receptors and a moderate affinity for 5-HT2C, D2, D3, 1- and 2B-adrenoceptors. This drug is a selective 5-HT2A, D1 and D4 antagonist. Extrapyramidal adverse effects also appear to be limited in pipamperone treatment compared to traditional antipsychotic medications due to its high receptor selectivity. Pipamperone has a 15-fold higher affinity for D4 than D2 receptors. It has been suggested that D4 receptors may play a role in the modulation of GABAergic neuronal activity by dopamine.