

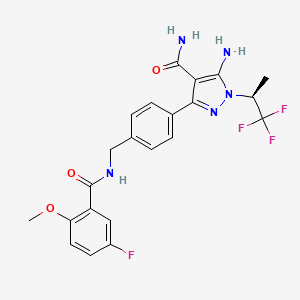

1. 5-amino-3-(4-(((5-fluoro-2-methoxybenzoyl)amino)methyl)phenyl)-1-((2s)-1,1,1-trifluoropropan-2-yl)pyrazole-4-carboxamide
2. Loxo-305
1. Loxo-305
2. 2101700-15-4
3. Pirtobrutinib [usan]
4. Jna39i7zvb
5. (s)-5-amino-3-(4-((5-fluoro-2-methoxybenzamido)methyl)phenyl)-1-(1,1,1-trifluoropropan-2-yl)-1h-pyrazole-4-carboxamide
6. Ly3527727
7. Rxc-005
8. Ly-3527727
9. 1h-pyrazole-4-carboxamide, 5-amino-3-(4-(((5-fluoro-2-methoxybenzoyl)amino)methyl)phenyl)-1-((1s)-2,2,2-trifluoro-1-methylethyl)-
10. 1h-pyrazole-4-carboxamide, 5-amino-3-[4-[[(5-fluoro-2-methoxybenzoyl)amino]methyl]phenyl]-1-[(1s)-2,2,2-trifluoro-1-methylethyl]-
11. Btk Inhibitor 16
12. Unii-jna39i7zvb
13. Pirtobrutinib [inn]
14. Pirtobrutinib [jan]
15. Loxo305
16. Pirtobrutinib (loxo-305)
17. Pirtobrutinib [who-dd]
18. Chembl4650485
19. Schembl19014257
20. Gtpl11628
21. Glxc-25733
22. Ex-a5016
23. Who 11681
24. Bl180882
25. Hy-131328
26. Cs-0133286
27. Loxo-305;ly 3527727; Rxc-005
28. 5-amino-3-(4-((5-fluoro-2-methoxybenzamido)methyl)phenyl)-1-((2s)-1,1,1-trifluoropropan-2-yl)-1h-pyrazole-4-carboxamide
29. 5-amino-3-[4-[[(5-fluoro-2-methoxybenzoyl)amino]methyl]phenyl]-1-[(2s)-1,1,1-trifluoropropan-2-yl]pyrazole-4-carboxamide
| Molecular Weight | 479.4 g/mol |
|---|---|
| Molecular Formula | C22H21F4N5O3 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 479.15805220 g/mol |
| Monoisotopic Mass | 479.15805220 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 719 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)