


1. Ampicillin Pivaloyl Ester
2. Berocillin
3. Ester, Ampicillin Pivaloyl
4. Hydrochloride, Pivampicillin
5. Monohydrochloride, Pivampicillin
6. Pivamiser
7. Pivampicillin Hydrochloride
8. Pivampicillin Monohydrochloride
9. Pondocillin
1. Pivaloylampicillin
2. 33817-20-8
3. Pivaloyloxymethyl Ampicillinate
4. Pivampicilina
5. Pivampicilline
6. Pivampicillinum
7. Ampicillin Pivaloyloxymethyl Ester
8. Pivampicilina [inn-spanish]
9. Pivampicilline [inn-french]
10. Pivampicillinum [inn-latin]
11. Pivampicillin (inn)
12. 0hlm346ll7
13. Chebi:8255
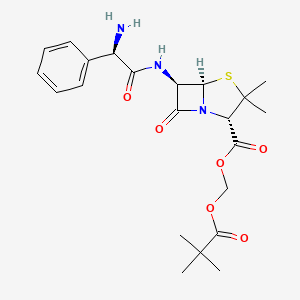
14. 2,2-dimethylpropanoyloxymethyl (2s,5r,6r)-6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
15. Mk-191
16. Pivampicillin [inn]
17. Dsstox_cid_25459
18. Dsstox_rid_80890
19. Dsstox_gsid_45459
20. 2,2-dimethylpropanoyloxymethyl (2s,5r,6r)-6-[(2-amino-2-phenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
21. Pivampicillin [inn:ban]
22. Mk 191
23. Pondocillin (tn)
24. Einecs 251-688-6
25. Unii-0hlm346ll7
26. Ncgc00016823-01
27. Cas-33817-20-8
28. Prestwick0_001009
29. Prestwick1_001009
30. Prestwick2_001009
31. Prestwick3_001009
32. Pivampicillin [mi]
33. Schembl34182
34. Bspbio_001137
35. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester, (2s-(2alpha,5alpha,6beta(s*)))-
36. Pivampicillin [mart.]
37. Spbio_003018
38. Pivampicillin [who-dd]
39. Bpbio1_001251
40. Chembl3182343
41. Dtxsid1045459
42. Hms1571i19
43. Hms2098i19
44. Hms3715i19
45. Pivampicillin [ep Monograph]
46. Tox21_110631
47. Zinc34967244
48. Tox21_110631_1
49. Ccg-221009
50. Db01604
51. Ncgc00179290-01
52. Ncgc00179290-03
53. [(2,2-dimethylpropanoyl)oxy]methyl 6beta-[(2r)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylate
54. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-, Hydroxymethyl Ester, Pivalate (ester), D-(-)-
55. Ab00513999
56. D08396
57. Sr-01000872693
58. Q3122143
59. Sr-01000872693-1
60. (2s,5r,6r)-pivaloyloxymethyl 6-((r)-2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
61. {[(2s,5r,6r)-6-[(2r)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-2-yl]carbonyloxy}methyl 2,2-dimethylpropanoate
62. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester (2s-(2.alpha.,5.alpha.,6.beta.(s*)))-
63. Hydroxymethyl D-(-)-6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate Pivalate (ester)
| Molecular Weight | 463.5 g/mol |
|---|---|
| Molecular Formula | C22H29N3O6S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 463.17770683 g/mol |
| Monoisotopic Mass | 463.17770683 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 774 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
or the treatment of respiratory tract infections (including acute bronchitis, acute exacerbations of chronic bronchitis and pneumonia); ear, nose and throat infections; gynecological infections; urinary tract infections (including acute uncomplicated gonococcal urethritis) when caused by non penicillinase-producing susceptible strains of the following organisms: gram-positive organisms, e.g., streptococci, pneumococci and staphylococci; gram-negative organisms, e.g., H. influenzae, N. gonorrhoeae, E. coli, P. mirabilis.
Pivampicillin is the pivaloyloxymethyl ester of (the semi-synthetic penicillin) ampicillin. It is an inactive pro-drug, which is converted during its absorption from the gastrointestinal tract to the microbiologically active ampicillin, together with formaldehyde and pivalic acid, by non-specific esterases present in most body tissues. Amounts in excess of 99% of the pivampicillin absorbed are converted to ampicillin within 15 minutes of absorption.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CA - Penicillins with extended spectrum
J01CA02 - Pivampicillin
Absorption
Absorbed following oral administration.
Approximately 1 hour.
Ampicillin (the active metabolite of pivampicillin) has a bactericidal action resulting from inhibition of cell wall mucopeptide biosynthesis.