


1. Amdinocillin Pivoxil
2. Fl 1039
3. Fl-1039
4. Fl1039
5. Hydrochloride, Pivmecillinam
6. Mecillinam Pivaloyl Ester
7. Pivaloyl Ester, Mecillinam
8. Pivamdinocillin
9. Pivmecillinam Hydrochloride
10. Pivoxil, Amdinocillin
11. Selexid
1. Amdinocillin Pivoxil
2. 32886-97-8
3. Pivmecilinamo
4. Pivmecillinamum
5. Selexid
6. Amdinocillin Pivoxil [usan]
7. Pivmecilinamo [inn-spanish]
8. Pivmecillinamum [inn-latin]
9. Ro 10-9071
10. Amdinocillin, Pivaloyloxymethyl Ester
11. Pivmecillinam (inn)
12. Pivmecillinam [inn]
13. 1wam1oq30b
14. Amdinocillin Pivoxil (usan)
15. Ro-10-9071
16. Chebi:51210
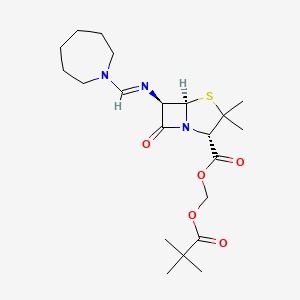
17. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester, (2s-(2alpha,5alpha,6beta))-
18. Hydroxymethyl (2s,5r,6r)-6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate Pivalate (ester)
19. Coactabs
20. [(2,2-dimethylpropanoyl)oxy]methyl (2s,5r,6r)-6-[(azepan-1-ylmethylidene)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
21. [(2,2-dimethylpropanoyl)oxy]methyl 6beta-[(azepan-1-ylmethylidene)amino]-2,2-dimethylpenam-3alpha-carboxylate
22. Coactabs (tn)
23. 2,2-dimethylpropanoyloxymethyl (2s,5r,6r)-6-(azepan-1-ylmethylideneamino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
24. Ncgc00016813-01
25. Cas-32887-03-9
26. Unii-1wam1oq30b
27. Sr-01000838842
28. Einecs 251-276-6
29. Prestwick0_001053
30. Prestwick1_001053
31. Prestwick2_001053
32. Prestwick3_001053
33. Pivmecillinam [jan]
34. Schembl33907
35. Schembl33908
36. Bspbio_001006
37. Pivmecillinam [mart.]
38. Spbio_002933
39. Pivmecillinam [who-dd]
40. Bpbio1_001108
41. Chembl1525183
42. Chembl1616433
43. Chembl1650818
44. Dtxsid7048538
45. Gtpl10922
46. Amdinocillin Pivoxil [mi]
47. Pivmecillinam, >=98% (hplc)
48. Bcp07843
49. Hy-b0810
50. Zinc4214799
51. Zinc13704173
52. Db01605
53. Ro-109071
54. Ncgc00179344-01
55. Ncgc00179344-04
56. Ncgc00179344-07
57. Ab00514713
58. D02889
59. A918810
60. Q418086
61. Sr-01000838842-2
62. Brd-k67100011-003-03-0
63. (2s,6r)-pivaloyloxymethyl 6-((e)-azepan-1-ylmethyleneamino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
64. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester, (2s-(2.alpha.,5.alpha.,6.beta.))-
| Molecular Weight | 439.6 g/mol |
|---|---|
| Molecular Formula | C21H33N3O5S |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 439.21409234 g/mol |
| Monoisotopic Mass | 439.21409234 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 710 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to treat infections due to mecillinam-sensitive organisms such as urinary tract infections, salmonellosis and typhoid fever.
Pivmecillinam is a pivaloyloxymethyl ester of amdinocillin that is well absorbed orally, but broken down to amdinocillin in the intestinal mucosa. It is active against gram-negative organisms and used as for amdinocillin.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CA - Penicillins with extended spectrum
J01CA08 - Pivmecillinam
Absorption
Well absorbed following oral administration.
Pivmecillinam interferes with the biosynthesis of the bacterial cell wall however its activity is slightly different from that of other penicillins and cephalosporins