


1. Mbri-001
2. Npi 2358
3. Npi-2358
4. Npi2358
1. 714272-27-2
2. Npi-2358
3. Plinabulin (npi-2358)
4. Plinabulin(npi-2358)
5. Npi-2358 (plinabulin)
6. Npi 2358
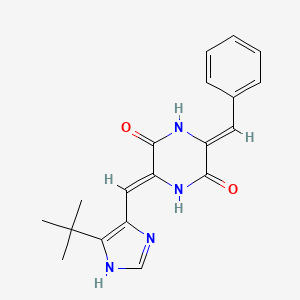
7. (3z,6z)-3-benzylidene-6-[(5-tert-butyl-1h-imidazol-4-yl)methylidene]piperazine-2,5-dione
8. 986fy7f8xr
9. Npi2358
10. (3e,6e)-3-benzylidene-6-[(5-tert-butyl-1h-imidazol-4-yl)methylidene]piperazine-2,5-dione
11. (3z,6z)-3-benzylidene-6-((5-(tert-butyl)-1h-imidazol-4-yl)methylene)piperazine-2,5-dione
12. 2,5-piperazinedione, 3-((5-(1,1-dimethylethyl)-1h-imidazol-4-yl)methylene)-6-(phenylmethylene)-, (3z,6z)-
13. Plinabulin [usan:inn]
14. Plinabulina
15. Plinabuline
16. Plinabulinum
17. Unii-986fy7f8xr
18. Plinabulin [mi]
19. Plinabulin [inn]
20. Plinabulin (usan/inn)
21. Plinabulin [usan]
22. Kpu-2
23. Plinabulin [who-dd]
24. Schembl79095
25. Mls006011262
26. Chembl1096380
27. Ex-a292
28. Chebi:177413
29. Dtxsid201031311
30. Bpi 2358
31. Bpi-2358
32. Zinc3819466
33. Bdbm50030765
34. Mfcd18074510
35. Nsc797934
36. S1176
37. Akos005145762
38. Akos024463284
39. Bcp9000994
40. Ccg-264849
41. Cs-0506
42. Db05992
43. Nsc-797934
44. Ac-32812
45. Hy-14444
46. Smr004703013
47. Bcp0726000116
48. Sw219827-1
49. D09655
50. Brd-k99498722-001-01-8
51. Q15269699
52. (3z,6z)-6-benzylidene-3-((5-(1,1-dimethylethyl)-1h-imidazol-4- Yl)methylidene)piperazine-2,5-dione
| Molecular Weight | 336.4 g/mol |
|---|---|
| Molecular Formula | C19H20N4O2 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 336.15862589 g/mol |
| Monoisotopic Mass | 336.15862589 g/mol |
| Topological Polar Surface Area | 86.9 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 597 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in cancer/tumors (unspecified).
NPI-2358 is a vascular disrupting agent currently in clinical development for the treatment of cancer by Nereus. NPI-2358 is one of over 200 synthetic analogues that were prepared following the discovery of the compound Halimide isolated from a marine fungus. In preclinical models of cancer, including lung, breast, sarcoma, colon and prostate, NPI-2358 demonstrated potent and selective anti-tumor effects in combination with docetaxel and other oncology therapies, as well as single-agent efficacy in a number of orthotopic models. NPI-2358 interacts with soluble beta-tubulin and prevents the polymerization of tubulin without altering dynamic microtubule function of formed microtubules. As demonstrated in preclinical testing, this target profile results in a highly specific nanomolar cytotoxicity while reducing the side effects seen in first-generation VDAs due to cardiotoxicity, hemodynamic changes and neuropathies.

LOOKING FOR A SUPPLIER?