


1. 7440-09-7
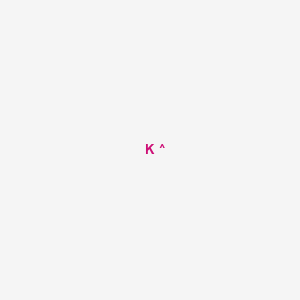
2. K
3. Mfcd00133776
4. Potassium, Metal
5. Monopotassium
6. Potasio
7. Mono-potassium
8. Potassium Atom
9. Potassium Cubes
10. Potassium Metal
11. Potassium, Elemental
12. Potassium, Saj First Grade
13. Potassium Hydride, In Paraffin
14. Chebi:26216
15. Potassium Metal, Cubes In Mineral Oil
16. Akos028109834
17. Db14500
18. Q703
19. Ft-0627093
20. Ft-0695235
21. Potassium, Ingot, 99.95% Trace Metals Basis
22. Potassium Hydride, 30 Wt % Dispersion In Mineral Oil
23. Potassium, Rod, Diam. 25 Mm, 99.5%, In Mineral Oil
24. Potassium, Solid, 99.95% Trace Metals Basis, Ampoule
25. Potassium, Chunks (in Mineral Oil), 98% Trace Metals Basis
26. Potassium, Oil Based Standard Solution, Specpure, K 1000 ?g/g
27. Potassium, Oil Based Standard Solution, Specpure?, K 5000?g/g
28. Potassium, Cubes (in Mineral Oil), L X W X H 40 Mm X 30 Mm X 20 Mm, 99.5% Trace Metals Basis
| Molecular Weight | 39.0983 g/mol |
|---|---|
| Molecular Formula | K |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 38.96370648 g/mol |
| Monoisotopic Mass | 38.96370648 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
**General uses of potassium** Potassium is indicated to treat a variety of conditions. Firstly, it used to replenish potassium that has been depleted by conditions including but not limited to malabsorption, decreased intake, or excess sodium intake. The causes of potassium deficiency are numerous. The following indications for potassium are not comprehensive, but include the main indications for which this nutrient is used. Various products and preparations contain potassium. **Potassium chloride** Potassium chloride is one of the main preparations of potassium used in a clinical setting. The oral solution is indicated for the prevention and treatment of hypokalemia presenting with or without metabolic alkalosis, in patients who have failed conservative management with potassium-rich foods or diuretic dose titrations. The injection form of potassium chloride is indicated to replenish potassium in patients who are not feasible candidates for oral potassium. Highly concentrated potassium is intended for the treatment of potassium deficiency in fluid restricted individuals who cannot tolerate fluid volumes normally associated with injected potassium solutions that contain lower concentrations. Finally, the extended-release tablet preparation of potassium chloride is used to treat hypokalemia with or without metabolic alkalosis, to treat digitalis intoxication, and to manage patients with hypokalemic familial periodic paralysis. It is also used in the prevention of hypokalemia in those who are at a high risk of negative clinical outcomes if hypokalemia occurs; patients on digitalis or those with cardiac arrhythmias would be at particular risk of negative outcomes. **Potassium chloride with dextrose and sodium chloride** This liquid preparation is is indicated in a clinical setting as a source of water, calories and electrolytes. Potassium acetate solution is meant as an alternative to potassium chloride, replenishing potassium and added to large volume infusion fluids for intravenous injection. **Potassium citrate** The potassium citrate preparation is used for the management of renal tubular acidosis (RTA) with calcium stones (nephrolithiasis); calcium oxalate stones by any cause, and uric acid nephrolithiasis (with or without calcium stones). This regimen also includes adequate water intake (leading to a urine out put of 2 L/day or more) and sodium restriction.
Potassium maintains an electrolyte gradient on cell surfaces, keeping at specific concentrations inside and outside of the cell; this impacts fluid and electrolyte balance, nerve transmission, muscle contraction, as well as cardiac and kidney function. Clinical evidence has associated potassium intake with lower blood pressure in adults, reducing the risk stroke and heart disease. Dietary potassium may exert beneficial effects on bone loss in the elderly and kidney stones. Consumption of white vegetables, which are normally high in potassium, is associated with a lower risk of stroke. **A note on gastrointestinal lesions** Potassium in solid oral preparations (for example, tablets) can cause ulcerative or stenotic lesions in the esophagus and stomach. Use diluted liquid potassium preparations or injection preparations if there are concerns about gastrointestinal health.
Absorption
When taken orally from a dietary source, potassium is mainly absorbed via passive diffusion in the small intestine. Approximately 90% of potassium is absorbed, and maintains concentrations both inside and outside cells. The kidneys can adapt to variable potassium intake in healthy individuals, but a minimum of 5 mmol (about 195 mg) dietary potassium is measured to be excreted in the urine. Some studies have measured the absorption various forms of potassium from dietary supplements. Results from a clinical trial in 2016 showed that potassium gluconate supplements are 94% absorbed, which is similar to the absorption rate from potatoes. An older study advised that liquid forms of potassium are absorbed a few hours post-administration. Enteric coated tablets of potassium chloride are not absorbed as rapidly as liquid forms, due to their delayed release design.
Route of Elimination
Potassium is excreted primarily in the urine, excreted in small amounts in the stool, and negligibly in perspiration (sweat). The renal system regulates potassium excretion according to dietary intake. Potassium excretion rises quickly in healthy patients after ingestion unless body stores have been depleted. Potassium undergoes glomerular filtration, tubular reabsorption, and distal tubular secretion. Renal clearance of potassium shifts between net tubular secretion and reabsorption, depending on the clinical circumstances.
Volume of Distribution
Potassium is present in almost all body tissues. Approximately 98% of potassium is maintained intracellularly in muscular tissue, the liver, and red blood cells. The remainder is distributed extracellularly.
Clearance
Potassium is freely filtered in the kidney with most of an ingested amount being reabsorbed into the circulation (70%80%) by the proximal tubule and loop of Henle. Secretion of potassium by the distal nephron in the kidney varies and dependent on the intracellular potassium concentration, luminal potassium concentration concentration, in addition to cellular permeability.
Potassium is absorbed and excreted in unchanged form.
In one clinical study, the apparent half-life of oral potassium was between 1.6 and 14 hours. A radio tracer study determined that the biological half-life of radiolabeled potassium ranges from 10 to 28 days.
Potassium ion is the primary intracellular cation found in virtually all body tissues. The total amount of body potassium in adults is estimated at 45 millimole (mmol)/kg body weight (about 140 g for an adult weighing 175 pounds; 1 mmol = 1 milliequivalent or 39.1 mg of potassium). Potassium mainly stays in cells, and a small amount can be found in the extracellular fluid. The amount of potassium that stays in the cell (intracellular) is 30 times that of extracellular concentration, creating a transmembrane gradient, regulated by the sodium-potassium (Na+/K+) ATPase transporter. This is an important gradient for nerve conduction, muscle contractions, and renal function. Vomiting, diarrhea, renal disease, medications, and other conditions that alter potassium excretion or shift it inside or outside of cells. In healthy patients individuals with normal renal function, markedly high or low potassium levels are rare. **Effect on blood pressure** Potassium decreases reduces intravascular volume, by reducing sodium reabsorption through an increase in urinary sodium excretion. This short-term effect, however, does not explain the long-term effects of potassium on blood pressure. Increased plasma potassium levels that occur through intake are associated with vasodilation occurring via stimulation of the sodium-potassium adenosine triphosphatase pump (Na+/-K+ATPase) and opening of potassium channels of the sodium-potassium adenosine triphosphatase pump. Other possible mechanisms of action for potassium may include alterations in barroreflex sensitivity and hormone sensitivity in vascular smooth muscle and cells of the sympathetic nervous system. **Effect on electrolyte balance and body systems** The potassium gradient across the membrane of a cell regulates cell membrane potential, maintained predominantly by the sodium-potassium (Na+/-K+ ATPase pump). Transmembrane electro-chemical gradients encourage diffusion of Na+ extracellularly and K+ intracellularly. Potassium supplementation prevents hypokalemia to maintain this balance and is often used in an oral solution or injection form in the clinical setting, preventing harmful effects such as arrhythmias, abnormal muscle function, and neurological disturbances. When activated, the Na+/-K+ ATPase pump exchanges two extracellular K+ ions for three intracellular sodium (Na+) ions, impacting membrane potential via either excitation or inhibition. This is especially important in the homeostasis of the nervous system, kidney, and cardiac muscle tissue. The body and cell distributions of potassium in normal conditions are known as internal and external balance, respectively. Reduced serum potassium (or imbalance) increases the risk of ventricular arrhythmia, heart failure and left ventricular hypertrophy (LVH).