

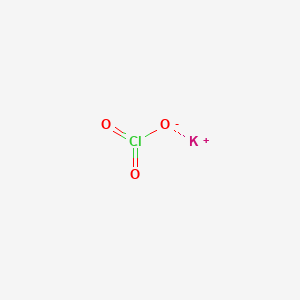

1. Chloric Acid
2. Chloric Acid, Potassium Salt
1. 3811-04-9
2. Potcrate
3. Anforstan
4. Fekabit
5. Potassium;chlorate
6. Kaliumchlorat
7. Mfcd00011361
8. H35ks68ee7
9. Chloric Acid Potassium Salt (1:1)
10. Salt Of Tarter
11. Chlorate Of Potassium
12. Potassium Chlorate(v)
13. Potash Chlorate (dot)
14. Chlorate De Potassium [french]
15. Hsdb 1110
16. Potassio (clorato Di) [italian]
17. Potassium (chlorate De) [french]
18. Postassium Chlorate
19. Einecs 223-289-7
20. Nsc 68505
21. Un1485
22. Un2427
23. Kali Chloricum
24. Potassio (clorato Di)
25. Kaliumchloraat [dutch]
26. Kaliumchlorat [german]
27. Ai3-02907
28. Dsstox_cid_27448
29. Dsstox_rid_82354
30. Unii-h35ks68ee7
31. Dsstox_gsid_47448
32. Kali Chloricum [hpus]
33. Potassium Chlorate(v) (htm)
34. K (cl O3)
35. Potassium Chlorate [mi]
36. Chembl3188561
37. Dtxsid6047448
38. Potassium Chlorate [hsdb]
39. Potassium Chlorate [inci]
40. Potassium Chlorate [mart.]
41. Potassium Chlorate [who-dd]
42. Potassium Chlorate, Aqueous Solution
43. Tox21_302523
44. Chloric Acid, Potassium Salt (1:1)
45. Akos015903479
46. Akos025293911
47. Ncgc00256721-01
48. Cas-3811-04-9
49. Potassium Chlorate [un1485] [oxidizer]
50. Ec 223-289-7
51. Q309328
52. Potassium Chlorate, Aqueous Solution [un2427] [oxidizer]
| Molecular Weight | 122.55 g/mol |
|---|---|
| Molecular Formula | ClKO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 121.9173030 g/mol |
| Monoisotopic Mass | 121.9173030 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 49.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
/VET/ In veterinary medicine, potassium chlorate is used as an oxidizing agent, antiseptic, and astringent ... . A 2 to 4% aqueous solution can be used as a weak astringent antiseptic in stomatitis and vaginitis; however, its effectiveness is questionable.
Cosmetic Ingredient Review Panel; J Am Coll Toxicol 14(3): 221-30 (1995).
Potassium chlorate was recommended for ulcerative stomatitis in humans for over 100 years, but it was dropped from the National Formulary in 1960 because of its lack of efficacy & potential toxicity.
National Research Council. Drinking Water and Health. Volume 3. Washington, DC: National Academy Press, 1980., p. 198
At one time, the chlorate salts, sodium chlorate and potassium chlorate, were used as medicinal agents to treat inflammatory and ulcerative lesions of the oral cavity and could be found in various mouthwash, toothpaste, and gargle preparations. /Former use/
Goldfrank, L.R., Goldfrank's Toxicologic Emergencies 8th Ed. 2006., McGraw-Hill, New York, N.Y., p. 1393
The lethal dose in adults is estimated to be...5 to 30 g for potassium chlorate.
National Research Council. Drinking Water and Health. Volume 3. Washington, DC: National Academy Press, 1980., p. 199
Toxic dose to a human is about 5 g.
Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 2756
... The lethal adult dose /is/ 15 to 35 g ... . Mortality of a child has been reported after ingestion of 1 g of potassium chlorate.
Cosmetic Ingredient Review Panel; J Am Coll Toxicol 14(3): 221-30 (1995).
The toxic effects of potassium chlorate appear to be cumulative because of slow excretion of the chlorate ion; repeated 1-g ingestions have been fatal ... .
Cosmetic Ingredient Review Panel; J Am Coll Toxicol 14(3): 221-30 (1995).
Two groups of Sprague-Dawley rats, four males per group, were dosed orally with 5 mg/L K(36)ClO3 (0.85 uCi) ... . For one group, blood samples were obtained by retro-orbital bleeding at various times for up to 48 hr after dosing. The animals were killed after 72 hr, with blood samples taken and various tissues collected. The (36)Cl content of the tissues was determined by liquid scintillation spectrometry. The second group of animals was placed in metabolism cages following dosing, with expired air and fecal and urine samples measured for up to 72 hr after dosing. The radioactivity of Cl-, ClO2-, and ClO3 was measured. For the first group of animals, a peak (36)Cl plasma concentration was reached after 30 min. Chlorate elimination from the plasma was two-phase; the rapid phase of elimination, the a-phase, had a half-life (t1/2) of 6 hr, and the slower phase of elimination, the beta-phase, had a t1/2 of 36.7 hr. Following ClO3- administration, measured radioactivity was greatest in the plasma, followed by the whole blood, stomach, testes, lungs, kidneys, skin, duodenum, spleen, brain, packed cells, ileum, carcass, liver, and bone marrow. The amount of (36)Cl in the plasma was approximately 2.0 ng/g, and in the bone marrow, less than 0.5 ng/g. For the second group of animals, about 43% of the chlorate was excreted in 72 hr; 39% of the (36)Cl was recovered in the urine and feces after 24 hr. No radioactivity was recovered in expired air. The administered ClO3- was eliminated as approximately 20% chloride, 4.0% chlorite, and 13.0% chlorate.
Cosmetic Ingredient Review Panel; J Am Coll Toxicol 14(3): 221-30 (1995).
Oral administration of 3 mL (5 mg/L) to rats have shown: absorption T1/2: 1.74 hr, elimination T1/2: 26.7 hr. Distribution mainly in blood, excretion in feces and urine: 43% of initial dose in 72 hr. Increased formation of chloroform in the liver.
European Chemicals Bureau; IUCLID Dataset, Potassium chlorate (3811-04-9) p.28 (2000 CD-ROM edition). Available from, as of July 31, 2008: https://esis.jrc.ec.europa.eu/
... Sprague-Dawley rats, four males per group, were dosed orally with 5 mg/L K(36)ClO3 (0.85 uCi) ... The administered ClO3- was eliminated as approximately 20% chloride, 4.0% chlorite, and 13.0% chlorate.
Cosmetic Ingredient Review Panel; J Am Coll Toxicol 14(3): 221-30 (1995).
Oral administration of 3 mL (5 mg/L) to rats have shown: absorption half life: 1.74 hr, elimination half life: 26.7 hr. .
European Chemicals Bureau; IUCLID Dataset, Potassium chlorate (3811-04-9) p.28 (2000 CD-ROM edition). Available from, as of July 31, 2008: https://esis.jrc.ec.europa.eu/