

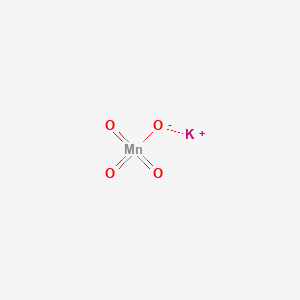

1. Permanganate, Potassium
1. 7722-64-7
2. Chameleon Mineral
3. Potassium Permanganate (kmno4)
4. Potassium Permanganate Solution
5. Potassium;permanganate
6. Kmno4
7. Mfcd00011364
8. 00ot1qx5u4
9. Permanganic Acid (hmno4), Potassium Salt (1:1)
10. Argucide
11. Walko Tablets
12. Algae-k
13. Solo San Soo
14. Pure Light E 2
15. Caswell No. 699
16. Diversey Diversol Cxu
17. Hilco #88
18. Kaliumpermanganat [german]
19. Potassium Permanganate [jan]
20. Permanganato Potasico
21. Ccris 5561
22. Permanganato Potasico [spanish]
23. Hsdb 1218
24. Diversey Diversol Cx With Arodyne
25. Permanganate De Potassium [french]
26. Einecs 231-760-3
27. Un1490
28. Potassio (permanganato Di) [italian]
29. Potassium (permanganate De) [french]
30. Epa Pesticide Chemical Code 068501
31. Nsc 146182
32. Unii-00ot1qx5u4
33. Ci 77755
34. Ai3-52835
35. Kali Permanganicum
36. Potassium Permanganate [usp:jan]
37. Potasiumpermanganate
38. Potassiumpermanganate
39. Icc 237 Disinfectant, Sanitizer, Destainer, And Deodorizer
40. Ec 231-760-3
41. Potassium Permanganate (tn)
42. Potassium Permanganate, 97%
43. Dtxsid2034839
44. Kali Permanganicum [hpus]
45. Potassium Permanganate [mi]
46. Potassium Permanganate, Acs Reagent
47. Potassium Permanganate (jp17/usp)
48. Potassium Permanganate [hsdb]
49. Potassium Permanganate [vandf]
50. Potassium Permanganate, Lr, >=99%
51. Akos015833392
52. Potassium Permanganate [mart.]
53. Potassium Permanganate Solution, 2 Mm
54. Potassium Permanganate Solution, 5 Mm
55. Db13831
56. Potassium Permanganate [who-dd]
57. Potassium Permanganate Solution, 0.1 M
58. Potassium Permanganate Solution, 0.2 M
59. Potassium Permanganate Solution, 0.25n
60. Potassium Permanganate, P.a., 99.0%
61. Potassium Permanganate Solution, 0.01 M
62. Potassium Permanganate Solution, 0.02 M
63. Ft-0645093
64. P1742
65. Potassium Permanganate [ep Monograph]
66. Potassium Permanganate [usp Monograph]
67. D02053
68. Potassium Permanganate [un1490] [oxidizer]
69. Potassium Permanganate, Acs Reagent, >=99.0%
70. Potassium Permanganate, Bioultra, >=99.0% (rt)
71. Q190865
72. Potassium Permanganate, 0.1n Standardized Solution
73. Potassium Permanganate, Saj First Grade, >=99.3%
74. Potassium Permanganate, Tested According To Ph.eur.
75. Potassium Permanganate, Jis Special Grade, >=99.3%
76. Potassium Permanganate, <=150 Mum Particle Size, 97%
77. Potassium Permanganate, Meets Usp Testing Specifications
78. Potassium Permanganate, Acs Reagent, >=99.0%, Low In Mercury
79. Potassium Permanganate, Low In Mercury (max. 0,005 Ppm Hg)
80. Potassium Permanganate, P.a., Acs Reagent, Reag. Iso, 99.0%
81. Potassium Permanganate, Purum P.a., >=99.0% (rt), Fine Crystals
82. Potassium Permanganate, Puriss. P.a., Acs Reagent, Reag. Ph. Eur., >=99%
83. Potassium Permanganate, Suitable For Determination Of Nitroxide, >=99.3%
84. Potassium Permanganate, Suitable For Determination Of Toxic Metals, >=99.5%
85. Potassium Permanganate, P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., 99.0-100.5%
86. Potassium Permanganate, Puriss. P.a., Acs Reagent, Hg <=0.000005%, >=99.0% (rt)
87. Potassium Permanganate, Puriss., Meets Analytical Specification Of Ph. Eur., Bp, Usp, 99-100.5%
| Molecular Weight | 158.034 g/mol |
|---|---|
| Molecular Formula | KMnO4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 157.881408 g/mol |
| Monoisotopic Mass | 157.881408 g/mol |
| Topological Polar Surface Area | 74.3 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 118 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
POTASSIUM PERMANGANATE, USP, HAS LIMITED TOPICAL EFFICACY AGAINST BACTERIA & FUNGI. CONCN OF 1:5000 OR MORE ARE NECESSARY FOR EFFECTIVE BACTERICIDAL ACTION, BUT THEY ARE IRRITATING TO TISSUES. ... SOLN OF 1:10,000 ARE USUALLY USED. HOWEVER, UP TO AN HR MAY BE REQUIRED TO KILL ... BACTERIA & SOME ... SURVIVE EXPOSURE TO THIS CONCN. FOR THIS REASON, PERMANGANATE HAS BEEN MADE OBSOLETE BY /OTHER/ ... DRUGS. BECAUSE OF THE ASTRINGENCY OF MANGANOUS & MANGANIC IONS ... SUBSTANCE IS OCCASIONALLY USED TO SUPPRESS VESICULAR STAGE OF ECZEMA-DERMATITIS. A WET DRESSING OF PERMANGANATE MAY BE EMPLOYED IN THE TREATMENT OF IVY POISONING.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 974
POTASSIUM PERMANGANATE IS EMPLOYED EXTERNALLY FOR GERMICIDAL, FUNGICIDAL, ASTRINGENT, OXIDIZING, & KERATOPLASTIC EFFECTS. SOLN ... SHOULD BE FRESHLY PREPARED.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 84:04
SOLN ... EMPLOYED IN DERMATOMYCOSES, ESPECIALLY TINEA PEDIS, TINEA CRURIS, & RINGWORM.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 998
DOSE: TOPICAL ... FOR IVY POISONING OR ECZEMA, 0.01%; FOR EPIDERMOPHYTOSIS, 1% ... DOSAGE FORMS: TABLETS FOR SOLN: 300 MG.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1111
For more Therapeutic Uses (Complete) data for POTASSIUM PERMANGANATE (8 total), please visit the HSDB record page.
POTASSIUM PERMANGANATE (0.025%-0.1%) HAS ASTRINGENT PROPERTIES BUT STAINS SKIN. TABLETS SHOULD BE COMPLETELY DISSOLVED IF PLACED IN TUB OF WATER, BECAUSE ... CAN PRODUCE CUTANEOUS NECROSIS IF PATIENT INADVERTENTLY SITS ON THEM.
American Medical Association, Department of Drugs. Drug Evaluations. 6th ed. Chicago, Ill: American Medical Association, 1986., p. 1027
MOST BACTERIA ARE KILLED WITHIN 1 HR BY PERMANGANATES IN DILUTION OF 1:10,000, BUT SOME MICROORGANISMS SURVIVE EXPOSURE TO MUCH HIGHER CONCN. GERMICIDAL EFFICIENCY ... IS GREATLY REDUCED IN PRESENCE OF ORG MATTER. CONCN STRONGER THAN 1:5000 ARE IRRITATING TO TISSUES. POTASSIUM PERMANGANATE, USP, FORMS DEEP PURPLE SOLN, BUT IT LEAVES A BROWN STAIN ... /IN TREATMENT OF SNAKEBITE/ ... SOME BELIEVE THAT CMPD DOES MORE HARM THAN GOOD IN THE CRUST THAT IS FORMED, WHICH PRECLUDES POSSIBILITY OF SUCTION & DRAINAGE FROM WOUND.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 998
Using potassium permanganate in "neutralizing" ingested nicotine, physostigmine, quinine, and strychnine is potentially dangerous. Potassium permanganate is a nephrotoxin and hepatotoxin, as well as a corrosive agent in the gastrointestinal tract. Aspiration can cause acute tracheobrochitis and bronchopneumonia. Fatty changes in the heart have been reported.
PMID:6338654 Decker WJ; Vet Hum Toxicol 25 (1): 13 (1983)
VET: PERMANGANATE SOLN DO NOT PENETRATE DEEPLY & HAVE ONLY SUPERFICIAL ACTION. /PERMANGANATES/
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 886
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AX - Other antiseptics and disinfectants
D08AX06 - Potassium permanganate
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB18 - Potassium permanganate
SYSTEMIC EFFECTS ARE NOT ... /COMMONLY SEEN/ BECAUSE OF POOR ABSORPTION.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-109
Adult channel catfish Ictalurus punctatus were exposed to waterborne potassium permanganate for 12 wk to determine if such exposure would alter the manganese content of axial muscle or liver tissue. Continuous exposure to 0.5 mg KMnO4 or exposure to 1 or 2 mg KMnO4/L on alternate days did not cause a significant incr in manganese in axial muscle or liver tissue. The mean (|SE) concn of manganese in axial muscle of unexposed controls was 0.262 | 0.018 mg/kg (wet weight). Means of scle could be detected within groups. The mean (|SE) concn of manganese in liver tissue of controls was 1.67 | 0.09 mg/kg (wet weight). Manganese concns in liver tissue of the three exposure groups were 1.57 | 0.07 mg/kg, 1.68 | 0.08 mg/kg, and 1.58 | 0.10 mg/kg, for 0.5 (continuous), 1, or 2 mg/L (alternate days), respectively. Manganese was thought to accumulate in liver tissue, however, there were no statistically significant differences between those groups & the controls.
Griffin BR et al.; Journal of Aquatic Animal Health 11 (3): 305-309 (1999)