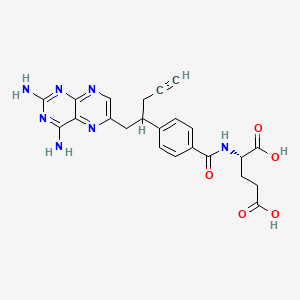

1. 10-propargyl-10-deazaaminopterin
1. 146464-95-1
2. Folotyn
3. 10-propargyl-10-deazaaminopterin
4. Hsdb 7786
5. Pdx
6. Pralatrexate(folotyn)
7. Chebi:71223
8. N-(4-(1-((2,4-diamino-6-pteridinyl)methyl)-3-butynyl)benzoyl)-l-glutamic Acid
9. Nsc-754230
10. (2s)-2-((4-((1rs)-1-((2,4-diaminopteridin-6-yl)methyl)but-3-ynyl)benzoyl)amino)pentanedioic Acid
11. (2s)-2-(4-(1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl)benzamido)pentanedioic Acid
12. A8q8i19q20
13. 146464-95-1 (racemic)
14. L-glutamic Acid, N-[4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]benzoyl]-
15. Ncgc00242596-01
16. (2s)-2-({4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl}amino)pentanedioic Acid
17. (2s)-2-[[4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl]amino]pentanedioic Acid
18. N-{4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl}-l-glutamic Acid
19. L-glutamic Acid, N-(4-(1-((2,4-diamino-6-pteridinyl)methyl)-3-butynyl)benzoyl)-
20. N -(4-{1-[(2,4-diaminopteridin-6-yl)methyl]but-3-yn-1-yl}benzoyl)-l-glutamic Acid
21. Folotyn (tn)
22. Pralatrexate [usan]
23. Pralatrexate [usan:inn]
24. Pralatrexato
25. Pralatrexatum
26. Pralatrexat
27. Unii-a8q8i19q20
28. Pralatrexate [mi]
29. Pralatrexate [inn]
30. Pralatrexate [jan]
31. Pralatrexate [hsdb]
32. Dsstox_cid_28504
33. Dsstox_rid_82776
34. Pralatrexate [vandf]
35. Dsstox_gsid_48578
36. Schembl21633
37. Pralatrexate [mart.]
38. Pralatrexate [who-dd]
39. Gtpl6840
40. Pralatrexate (jan/usan/inn)
41. Chembl1201746
42. Dtxsid3048578
43. Schembl15075302
44. Bcpp000101
45. Pralatrexate [orange Book]
46. Ex-a2142
47. Tox21_112906
48. Bdbm50457437
49. Mfcd00920897
50. Nsc754230
51. S1497
52. Akos015966891
53. Am84423
54. Ccg-269517
55. Cs-0504
56. Db06813
57. Nsc 754230
58. S11194
59. 10-propargyl-10-deazaaminopterin, 95%
60. Ncgc00386226-01
61. Ac-28388
62. Bs-15438
63. Hy-10446
64. Cas-146464-95-1
65. P2645
66. Sw220187-1
67. D05589
68. Ab00443251-02
69. Ab00443251_04
70. 464p951
71. A884505
72. Q637059
73. Sr-01000941578
74. J-008227
75. Sr-01000941578-1
76. N-(4-(1-((2,4-diamino-6-pteridinyl)methyl)-3-butynyl)benzoyl)-l-glutamic Acid;pralatrexate
| Molecular Weight | 477.5 g/mol |
|---|---|
| Molecular Formula | C23H23N7O5 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 10 |
| Exact Mass | 477.17606686 g/mol |
| Monoisotopic Mass | 477.17606686 g/mol |
| Topological Polar Surface Area | 207 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 809 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Folotyn |
| PubMed Health | Pralatrexate (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | FOLOTYN (pralatrexate injection) contains pralatrexate, which is an antineoplastic folate analog. Pralatrexate has the chemical name (2S)-2-[[4-[(1RS)-1-[(2, 4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioic acid. The structural for... |
| Active Ingredient | Pralatrexate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 40mg/2ml (20mg/ml); 20mg/ml (20mg/ml) |
| Market Status | Prescription |
| Company | Allos |
| 2 of 2 | |
|---|---|
| Drug Name | Folotyn |
| PubMed Health | Pralatrexate (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | FOLOTYN (pralatrexate injection) contains pralatrexate, which is an antineoplastic folate analog. Pralatrexate has the chemical name (2S)-2-[[4-[(1RS)-1-[(2, 4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioic acid. The structural for... |
| Active Ingredient | Pralatrexate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 40mg/2ml (20mg/ml); 20mg/ml (20mg/ml) |
| Market Status | Prescription |
| Company | Allos |
Aminopterin/ analogs & derivatives; Folic Acid Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Pralatrexate is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is based on overall response rate. Clinical benefit such as improvement in progression free survival or overall survival has not been demonstrated. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
T-cell lymphomas (TCL) are characterized by poor response to chemotherapy and generally poor outcome. While molecular profiling has identified distinct biological subsets and therapeutic targets in B-cell lymphomas, the molecular characterization of TCL has been slower. Surface markers expressed on malignant T-cells, such as CD2, CD3, CD4, CD25, and CD52 were the first TCL-specific therapeutic targets to be discovered. However, the presence of these receptors on normal T-cells means that monoclonal antibody (mAb)- or immunotoxin (IT)-based therapy in TCL inevitably results in variable degrees of immunosuppression. Thus, although some mAbs/IT have significant activity in selected subsets of TCL, more specific agents that target signaling pathways preferentially activated in malignant T-cells are needed. One such novel class of agents is represented by the histone deacetylase (HDAC) inhibitors. These molecules selectively induce apoptosis in a variety of transformed cells, including malignant T-cells, both in vitro and in vivo. Several HDAC inhibitors have been studied in TCL with promising results, and have recently been approved for clinical use. Immunomodulatory drugs, such as interferons and Toll Receptor (TLR) agonists have significant clinical activity in TCL, and are particularly important in the treatment of primary cutaneous subtypes (CTCL). Although most classical cytotoxic drugs have limited efficacy against TCL, agents that inhibit purine and pyrimidine metabolism, known as nucleoside analogues, and novel antifolate drugs, such as pralatrexate, are highly active in TCL. With improved molecular profiling of TCL novel pharmacological agents with activity in TCL are now being discovered at an increasingly rapid pace. Clinical trials are in progress and these agents are being integrated in combination therapies for TCL, both in the relapsed/refractory setting as well as front line.
PMID:20196721 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953129 Erter J et al; Curr Drug Targets (2009) (Epub ahead of print) .
FOLOTYN can suppress bone marrow function, manifested by thrombocytopenia, neutropenia, and anemia. Dose modifications are based on ANC and platelet count prior to each dose.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
Treatment with FOLOTYN may cause mucositis. If /greater than or equal to/ Grade 2 mucositis is observed, dose should be modified.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
Patients should be instructed to take folic acid and receive vitamin B12 to potentially reduce treatment-related hematological toxicity and mucositis. ... Patients should take low-dose oral folic acid on a daily basis. Folic acid should be initiated during the 10-day period preceding the first dose of FOLOTYN, and dosing should continue during the full course of therapy and for 30 days after the last dose of FOLOTYN. Patients should also receive a vitamin B12 intramuscular injection no more than 10 weeks prior to the first dose of FOLOTYN and every 8-10 weeks thereafter. Subsequent vitamin B12 injections may be given the same day as treatment with FOLOTYN.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
Although FOLOTYN has not been formally tested in patients with renal impairment, caution is advised when administering FOLOTYN to patients with moderate to severe impairment. Monitor patients for renal function and systemic toxicity due to increased drug exposure.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
For more Drug Warnings (Complete) data for Pralatrexate (10 total), please visit the HSDB record page.
Treatment of relapsed or refractory peripheral T-cell lymphoma.
FDA Label
treatment of peripheral T-cell lymphoma,
Pralatrexate is a 10-deazaaminopterin analogue of methotrexate. Compared to methotrexate, pralatrexate binds to RTC-1 with 10-times the affinity and is a more potent substrate for FPGS. As a result, pralatrexate is better internalized and retained in cancer cells and is more cytotoxic. Km, pralatrexate = 0.3 mol/L; Km, methotrexate = 4.8 mol/L; Vmax/Km (rate of intracellular transport), pralatrexate = 12.6 Vmax/Km (rate of intracellular transport), methotrexate = 0.9
L01BA05
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BA - Folic acid analogues
L01BA05 - Pralatrexate
Absorption
Pralatrexate demonstrates linear pharmacokinetics with a multiphasic decline with both diasteromers over dose range of 30-325 mg/m^2. Bioavailability, nonformulated preparation = 13 - 20%
Route of Elimination
35% of drug is excreted unchanged in the urine (no difference between R- and S- pralatrexate). May be some net renal tubular excretion.
Volume of Distribution
Vss, R-pralatrexate = 37 L Vss, S-pralatrexate = 105 L
Clearance
R- pralatrexate = 191 mL/min S- pralatrexate = 417 mL/min Mean clearance of both enantiomers is 220 mL/min.
The pharmacokinetics of pralatrexate administered as a single agent at a dose of 30 mg/sq m administered as an intravenous push over 3-5 minutes once weekly for 6 weeks in 7-week cycles have been evaluated in 10 patients with PTCL. The total systemic clearance of pralatrexate diastereomers was 417 mL/min (S-diastereomer) and 191 mL/min (R-diastereomer).
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
Pralatrexate total systemic exposure (AUC) and maximum plasma concentration (Cmax) increased proportionally with dose (dose range 30-325 mg/sq m, including pharmacokinetics data from high dose solid tumor clinical studies). The pharmacokinetics of pralatrexate did not change significantly over multiple treatment cycles, and no accumulation of pralatrexate was observed.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
Pralatrexate diastereomers showed a steady-state volume of distribution of 105 L (S-diastereomer) and 37 L (R-diastereomer).
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
In vitro studies indicate that pralatrexate is approximately 67% bound to plasma proteins.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
For more Absorption, Distribution and Excretion (Complete) data for Pralatrexate (7 total), please visit the HSDB record page.
No involvement of CYP450 enzyme system or glucuronidases.
In vitro studies using human hepatocytes, liver microsomes and S9 fractions, and recombinant human CYP450 isozymes showed that pralatrexate is not significantly metabolized by the phase I hepatic CYP450 isozymes or phase II hepatic glucuronidases.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
12-18 hours
The terminal elimination half-life of pralatrexate was 12-18 hours (coefficient of variance (CV) = 62-120%).
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
The selectivity of pralatrexate for cancer cells is based upon the observation that cancer cells generally have an overexpression of reduced folate carrier protein-1 (RTC-1) compared to normal somatic cells. This carrier protein allows the entrance of pralatrexate into the cell. Upon entering the cell, folypolyglutamate synthase FPGS catalyzes the polyglutamination of pralatrexate so that it is retained inside the cell. Once inside, pralatrexate competitively inhibits dihydrofolate reductase (DHFR) and thymidylate synthase. Subsequent depletion of thymidine monophosphate (TMP) occurs so that the cancer cell is unable to synthesize DNA and RNA. As a result, the cancer cell cannot proliferate and is forced to undergo apoptosis. Pralatrexate is more effective against cells that are actively dividing.
Pralatrexate is a folate analogue metabolic inhibitor that competitively inhibits dihydrofolate reductase. It is also a competitive inhibitor for polyglutamylation by the enzyme folylpolyglutamyl synthetase. This inhibition results in the depletion of thymidine and other biological molecules the synthesis of which depends on single carbon transfer.
US Natl Inst Health; DailyMed. Current Medication Information for FOLOTYN (pralatrexate) injection (September 2009). Available from, as of March 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11995
This study evaluated mechanistic differences of pralatrexate, methotrexate, and pemetrexed. Inhibition of dihydrofolate reductase (DHFR) was quantified using recombinant human DHFR. Cellular uptake and folylpolyglutamate synthetase (FPGS) activity were determined using radiolabeled pralatrexate, methotrexate, and pemetrexed in NCI-H460 non-small cell lung cancer (NSCLC) cells. The tumor growth inhibition (TGI) was assessed using MV522 and NCI-H460 human NSCLC xenografts. Apparent K ( i ) values for DHFR inhibition were 45, 26, and >200 nM for pralatrexate, methotrexate, and pemetrexed, respectively. A significantly greater percentage of radiolabeled pralatrexate entered the cells and was polyglutamylatated relative to methotrexate or pemetrexed. In vivo, pralatrexate showed superior anti-tumor activity in both NSCLC models, with more effective dose-dependent TGI in the more rapidly growing NCI-H460 xenografts. Pralatrexate demonstrated a distinct mechanistic and anti-tumor activity profile relative to methotrexate and pemetrexed. Pralatrexate exhibited enhanced cellular uptake and increased polyglutamylation, which correlated with increased TGI in NSCLC xenograft models.
PMID:19221750 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2728224 Izbicka E et al; Cancer Chemother Pharmacol 64 (5): 993-9 (2009).