

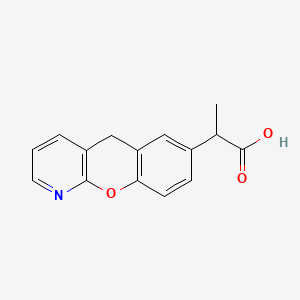

1. 2-(5h-(1)benzopyrano(2,3-b)pyridin-7-yl)propionic Acid
2. Alpha-methyl-5h-(1)benzopyrano(2,3-b)pyridine-7-acetic Acid
3. Niflan
4. Nifran
5. Oftalar
6. Pyranoprofen
7. Pyranoprofen, Methyl-14c-labeled
8. Zaltoprofen
1. 52549-17-4
2. Niflan
3. Pyranoprofen
4. 2-(5h-chromeno[2,3-b]pyridin-7-yl)propanoic Acid
5. Elicapric
6. Y-8004
7. Nsc-759832
8. 2-{5h-chromeno[2,3-b]pyridin-7-yl}propanoic Acid
9. 2-(5h-(1)benzopyrano(2,3-b)pyridin-7-yl)propionic Acid
10. Chembl367463
11. 2r7o1et613
12. 2-(5h-[1]benzopyrano[2,3-b]pyridin-7-yl)propionic Acid
13. Ncgc00167980-01
14. Dsstox_cid_3497
15. Dsstox_rid_77055
16. Dsstox_gsid_23497
17. Pranoprofene
18. Pranoprofeno
19. Pranoprofenum
20. Pranoprofen [inn:jan]
21. Pranoprofene [inn-french]
22. Pranoprofenum [inn-latin]
23. Pranoprofeno [inn-spanish]
24. Cas-52549-17-4
25. Sr-05000001513
26. Brn 0889798
27. Pransus
28. Unii-2r7o1et613
29. Pranoprofen;
30. Elicapric (tn)
31. 2-(5h-(1)benzopyrano(2,3-b)pyridin-7yl)propionic Acid
32. Alpha-methyl-5h-(1)-benzopyrano(2,3-b)pyridine-7-acetic Acid
33. Alpha-methyl-5h-(1)benzopyrano(2,3-b)pyridine-7-acetic Acid
34. Pranoprofen [mi]
35. Pranoprofen [inn]
36. Pranoprofen [jan]
37. Pranoprofen (jp17/inn)
38. Pranoprofen [mart.]
39. Schembl26008
40. Pranoprofen [who-dd]
41. Dtxsid1023497
42. Chebi:32040
43. Pranoprofen, >=98% (hplc)
44. (2s)-2-(5h-chromeno[2,3-b]pyridin-7-yl)propanoic Acid
45. Hms2090j03
46. Hms3651g06
47. Hms3714i03
48. Hms3884o16
49. Pharmakon1600-01502324
50. 5h-(1)benzopyrano(2,3,-b)pyridine-7-acetic Acid, Alpha-methyl-
51. Bcp11947
52. Ex-a5741
53. Hy-b0336
54. Tox21_112601
55. 5h-[1]benzopyrano[2,3-b]pyridine-7-acetic Acid, .alpha.-methyl-
56. Ac-697
57. Bdbm50286819
58. Mfcd00866111
59. Nsc759832
60. S1960
61. Akos015918442
62. Tox21_112601_1
63. Ccg-213822
64. Db13514
65. Nsc 759832
66. Ncgc00167980-02
67. As-12631
68. Ft-0652901
69. P2778
70. Sw199147-2
71. D01578
72. F17350
73. Ab01275517-01
74. Ab01275517_02
75. Ab01275517_03
76. 2-(5h-chromeno[2,3-b]pyridin-7-yl)propanoicacid
77. 549p174
78. Q-201611
79. Q7238394
80. Sr-05000001513-1
81. Sr-05000001513-2
82. Brd-a64610707-001-01-0
83. I2-(10h-9-oxa-1-aza-anthracen-6-yl)-propionic Acid
84. 2-(5h-[1]benzopyrano-[2,3-b]pyridin-7-yl)propionic Acid
85. .alpha.-methyl-5h-(1)benzopyrano(2,3-b)pyridine-7-acetic Acid
| Molecular Weight | 255.27 g/mol |
|---|---|
| Molecular Formula | C15H13NO3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 255.08954328 g/mol |
| Monoisotopic Mass | 255.08954328 g/mol |
| Topological Polar Surface Area | 59.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 346 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
S01BC09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC09 - Pranoprofen
