
1. 640315, Ly
2. 747, Cs
3. Cs 747
4. Cs-747
5. Cs747
6. Effient
7. Efient
8. Hcl, Prasugrel
9. Hydrochloride, Prasugrel
10. Ly 640315
11. Ly-640315
12. Ly640315
13. Prasugrel Hcl
14. Prasugrel Hydrochloride
1. 150322-43-3
2. Effient
3. Efient
4. Cs-747
5. Prasugrel (effient)
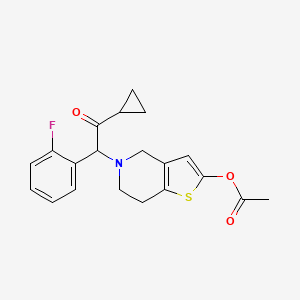
6. 5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl Acetate
7. Ly-640315
8. Ly640315
9. Cs 747
10. Pcr 4099
11. 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl Acetate
12. Chebi:87723
13. 34k66tbt99
14. Nsc-759625
15. 2-[2-(acetyloxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl]-1-cyclopropyl-2-(2-fluorophenyl)ethanone
16. [5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-6,7-dihydro-4h-thieno[3,2-c]pyridin-2-yl] Acetate
17. 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4h,5h,6h,7h-thieno[3,2-c]pyridin-2-yl Acetate
18. Ethanone, 2-[2-(acetyloxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl]-1-cyclopropyl-2-(2-fluorophenyl)-
19. Prasita
20. Prasugrel [inn]
21. Ethanone, 2-(2-(acetyloxy)-6,7-dihydrothieno(3,2-c)pyridin-5(4h)-yl)-1-cyclopropyl-2-(2-fluorophenyl)-
22. Smr002533665
23. Unii-34k66tbt99
24. Prasugrel [inn:ban]
25. Ncgc00188690-01
26. Ncgc00188690-02
27. Hsdb 7995
28. Prasugrel- Bio-x
29. Prasugrel-[d5]
30. 2-(2-(acetyloxy)-6,7-dihydrothieno(3,2-c)pyridin-5(4h)-yl)-1-cyclopropyl-2-(2-fluorophenyl)ethanone
31. 5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno(3,2-c)pyridin-2-yl Acetate
32. Prasugrel [mi]
33. Prasugrel [vandf]
34. Prasugrel [who-dd]
35. Prasugrel [ema Epar]
36. Mls003882595
37. Mls006012001
38. Schembl245032
39. Gtpl7562
40. Chembl1201772
41. Prasugrel, >=98% (hplc)
42. Schembl14112007
43. Dtxsid70861544
44. Hms3604b09
45. Hms3654d09
46. Hms3884i07
47. Act06208
48. Bcp01882
49. Fd7194
50. Mfcd09954140
51. S1258
52. Stl232602
53. Zb0747
54. Akos015841187
55. Ac-1640
56. Ccg-268338
57. Cs-0657
58. Db06209
59. Ks-5301
60. Nsc 759625
61. Pb23765
62. Sb20805
63. Ncgc00188690-03
64. [5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxo-ethyl]-6,7-dihydro-4h-thieno[3,2-c]pyridin-2-yl] Acetate
65. 2-acetoxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
66. Bp164290
67. Hy-15284
68. Am20090728
69. Ft-0650150
70. P2040
71. Sw219175-1
72. Ab01274761-01
73. Ab01274761_02
74. 322p433
75. A809033
76. Ar-270/43507998
77. Q416232
78. Q-101872
79. [5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-6,7-dihydro-4h-thieno[4,5-c]pyridin-2-yl] Acetate
80. 2-acetoxy-5-(.alpha.-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno-(3,2-c)pyridine
81. 2-acetoxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c] Pyridine
82. 2-acetoxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)4,5,6,7-tetrahydrothieno[3,2-c]pyridine
83. 2-acetyloxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
84. 2-acetyloxy-5-(alpha-cycloproylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
85. 5-((1rs)-2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno(3,2-c)pyridin-2-yl Acetate
86. 5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-ylacetate
87. Acetic Acid 5-[2-cyclopropyl-1-(2-fluoro-phenyl)-2-oxo-ethyl]-4,5,6,7-tetrahydro-thieno[3,2-c]pyridin-2-yl Ester
| Molecular Weight | 373.4 g/mol |
|---|---|
| Molecular Formula | C20H20FNO3S |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 373.11479284 g/mol |
| Monoisotopic Mass | 373.11479284 g/mol |
| Topological Polar Surface Area | 74.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 555 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Effient |
| PubMed Health | Prasugrel (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Active Ingredient | Prasugrel hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Eli Lilly And |
| 2 of 2 | |
|---|---|
| Drug Name | Effient |
| PubMed Health | Prasugrel (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Active Ingredient | Prasugrel hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Eli Lilly And |
Purinergic P2Y Receptor Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Prasugrel hydrochloride is used in combination with aspirin for the reduction of thrombotic cardiovascular events (e.g., stent thrombosis, myocardial infarction in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Prasugrel is used in patients with unstable angina or non-ST-segment-elevation myocardial infarction undergoing percutaneous coronary intervention and in patients with ST-segment elevation myocardial infarction managed with primary or nonprimary/delayed (i.e., after medical treatment for ST-segment elevation myocardial infarction) percutaneous coronary intervention. Because of established cardiovascular benefits, dual antiplatelet therapy with aspirin and prasugrel or clopidogrel is part of the current standard of care in patients with acute coronary syndromes. /Included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1551
/BOXED WARNING/ WARNING: BLEEDING RISK. Effient can cause significant, sometimes fatal, bleeding. Do not use Effient in patients with active pathological bleeding or a history of transient ischemic attack or stroke. In patients > or = 75 years of age, Effient is generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior myocardial infarction (MI)) where its effect appears to be greater and its use may be considered. Do not start Effient in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue Effient at least 7 days prior to any surgery. Additional risk factors for bleeding include: body weight < 60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding (e.g., warfarin, heparin, fibrinolytic therapy, chronic use of non-steroidal anti-inflammatory drugs [NSAIDS]). Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of Effient. If possible, manage bleeding without discontinuing Effient. Discontinuing Effient, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular events.
US Natl Inst Health; DailyMed. Current Medication Information for EFFIENT (prasugrel hydrochloride) tablet, film coated (September 2011). Available from, as of October 18, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fe9c118-c44b-48d7-a142-9668ae3df0c6
Geriatric patients, particularly those 75 years of age and older, appear to be at greater risk of bleeding (including fatal bleeding) with prasugrel therapy compared with younger patients. In the TRITON-TIMI 38 trial, about 39% of patients were 65 years of age or older and 13% were 75 years of age or older. Risk of bleeding increased with advancing age in both prasugrel and clopidogrel treatment groups. Among patients 75 years of age or older, fatal bleeding was more common with prasugrel than with clopidogrel (1 or 0.1%, respectively); symptomatic intracranial hemorrhage also was reported more frequently with prasugrel (0.8 or 0.3%, respectively). Mean exposure to the active metabolite of prasugrel is approximately 19% higher in patients 75 years or older compared with younger patients. Prasugrel generally should be avoided in patients 75 years of age or older because of a higher risk of bleeding and uncertain efficacy, but use may be considered in certain patients with high-risk conditions (e.g., diabetes, previous MI) in whom a greater net clinical benefit has been demonstrated.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1553
Thienopyridines, including prasugrel, should be discontinued prior to elective surgery. In patients who require elective coronary artery bypass grafting (CABG), discontinuance of prasugrel therapy is recommended at least 7 days prior to surgery. Prasugrel should not be initiated in those who are likely to undergo emergent CABG. To minimize the risk of adverse cardiac events, thienopyridines, including prasugrel, and other antiplatelet therapy should be resumed as soon as possible after temporary discontinuance of therapy for adverse effects or invasive procedures.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1552
Safety and efficacy have not been established in pediatric patients.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1553
For more Drug Warnings (Complete) data for Prasugrel (14 total), please visit the HSDB record page.
Indicated in combination with acetylsalicylic acid (ASA) to prevent atherothrombotic events in patients with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI). May be used in patients with unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI), ST-elevation myocardial infarction (STEMI) who are to be managed with PCI. Prasugrel is not recommended in patients 75 years of age or greater, those that weigh<60kg, and patients with a history of stroke or transient ischemic attack due to increased risk of fatal and intracranial bleeding.
FDA Label
Efient, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in patients with acute coronary syndrome (i. e. unstable angina, non-ST-segment-elevation myocardial infarction [UA / NSTEMI] or ST-segment-elevation myocardial infarction [STEMI]) undergoing primary or delayed percutaneous coronary intervention (PCI).
Prasugrel is a thienopyridine ADP receptor inhibitors which inhibits platelet aggregation by irreversibly binding to P2Y12 receptors.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B01AC22
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC22 - Prasugrel
Absorption
79% or greater of the dose is absorbed after oral administration. Absorption and metabolism occur rapidly and peak plasma concentrations (Cmax) are reached approximately 30 minutes following oral administration. Administration with a high fat, high calorie meal did not affect the AUC of the active metabolite in healthy individuals, but the Cmax was decreased by ~49% and the Tmax was increased to 0.5 to 1.5 hours. Prasugrel may be administered with or without food.
Route of Elimination
Approximately 68% of the orally administered dose is excreted in urine and 27% in the feces, as inactive metabolites. The active metabolite is not expected to be removed by dialysis.
Volume of Distribution
44-68L
Clearance
Apparent clearance = 112 - 166 L/hr
Tissue distribution of radioactivity related to prasugrel was studied in rats following single and repeated oral administration. Radioactivity was widely and rapidly distributed throughout the body. The radioactivity concentration was highest in most tissues involved in the absorption and elimination of the compound and its metabolites, i.e., stomach, intestines, liver, kidney and urinary bladder. Prasugrel distributed to the bone marrow of rats with a tissue-to-plasma ratio of less than 0.5. Following repeated daily dosing, accumulation consistent with the elimination half-life of prasugrel was observed in most organs.
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.12 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
Prasugrel is rapidly absorbed in all species including humans; Tmax of the active metabolite R-138727 is less than 1 hour. Prasugrel itself was not detected in plasma after oral administration. The decline of prasugrel related radioactivity was biphasic in rats and dogs. The radioactivity terminal elimination half-life seemed to be similar in mice and rats, approximately 24 hr, but it is considerably longer in dogs, approximately 3 days. In humans, the average terminal elimination half-life of the active metabolite R-138727 was approximately 7 hours. Approximately 21% of a (14)C-prasugrel dose is excreted in human feces within 48 hours.
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.12 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
In mice, 90% of the dose was excreted during the first 24 hours post dosing mainly via the urinary elimination route. In rats and dogs, the majority of the radioactivity (>90%) was excreted within the first 72 hours of dosing in faeces presumably via bile. Approximately 20% of the dose was excreted via urine. Radioactivity related to prasugrel was also detected in milk of lactating rats at concentrations up to approximately five times higher than the plasma level. However, the radioactivity from milk (half life =9.5 h) was eliminated more rapidly than that from the plasma (half life approximately 24 hr).
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.13 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
After a single oral dose of 5 mg/kg 14C-prasugrel to rats on Day 13 of pregnancy, the fetal concentration of prasugrel radioactivity was 0.27 times that in maternal blood 1 hour after administration and declined thereafter, suggesting low placental transfer of prasugrel or its metabolites.
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.13 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to thiolactone by human carboxylesterase (hCE) 2. This intermediate is further metabolized to its active metabolite, R-138727, in a single step by cytochrome P450 enzymes in the liver (primarily CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19). The active metabolite is further metabolized by S-methylation or cysteine conjugation to two inactive metabolites. Unlike clopidogrel, transformation of prasugrel to its active metabolite does not appear to be affected by cytochrome P450 polymorphisms.
Prasugrel was extensively metabolised in all species. A total of eighteen metabolites were identified in human plasma. Based on a mean radioactivity above 10%, the following major metabolites could be identified: diastereomers of M1, M2 (R-95913) and M5 (R-106583). The metabolites of prasugrel found in human plasma, urine and faeces were also detected in mouse, rat and dog; the only exception being M16, which was only identified in the mouse. M16 is M10 conjugated to glucuronic acid and M10 was found in all species. Furthermore, the extent of formation of a given metabolite varied significantly by species. Metabolite M1 was formed in large amounts in humans and was detected in animal plasma, but quantification was not conducted in animals due to co-eluting of the radioactive peaks. Metabolites M2, M5, M7 and M14 were also formed in larger amounts in humans as compared to the animal species.
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.13 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel's active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins. Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for EFFIENT (prasugrel hydrochloride) tablet, film coated (September 2011). Available from, as of October 18, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Prasugrel
When administered at high doses ( > or = 100 mg/kg) to rats, prasugrel induced CYP450 enzymes (CYP2B and CYP3A2) and phase II metabolizing enzymes UDP-glucuronosyltransferase and glutathione- S-transferase, however, based on the in vitro non clinical study with human hepatocytes, this induction is not observed in humans. Furthermore, the AUC for each measured metabolite decreased after multiple dosing compared with the values obtained after the first dose in mice at > or = 100 mg/kg/day, in rats at 100 and 300 mg/kg/day, and in dogs at 20 mg/kg/day (after 20 weeks of dosing and beyond). However, the exposure data in dogs administered prasugrel at 20 mg/kg for one month were essentially unchanged. In the nine month study with dogs, the AUC data for two of the metabolites R-100932 and R-106583 decreased after 20 weeks of dosing, while the AUC values of the other metabolite, R-95913 were higher in dogs dosed with prasugrel at 20 mg/kg. Thus, the data show some auto-induction of prasugrel's metabolism at the 20-mg/kg dose in dogs.
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.13 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
Prasugrel ... undergoes rapid hydrolysis in vivo to a thiolactone, R-95913, which is further converted to its thiol-containing, pharmacologically active metabolite, R-138727, by oxidation via cytochromes P450 (P450). We trapped a sulfenic acid metabolite as a mixed disulfide with 2-nitro-5-thiobenzoic acid in an incubation mixture containing the thiolactone R-95913, expressed CYP3A4, and NADPH. Further experiments investigated one possible mechanism for the conversion of the sulfenic acid to the active thiol metabolite in vitro. A mixed disulfide form of R-138727 with glutathione was found to be a possible precursor of R-138727 in vitro when glutathione was present. The rate constant for the reduction of the glutathione conjugate of R-138727 to R-138727 was increased by addition of human liver cytosol to the human liver microsomes. Thus, one possible mechanism for the ultimate formation of R-138727 in vitro can be through formation of a sulfenic acid mediated by P450s followed possibly by a glutathione conjugation to a mixed disulfide and reduction of the disulfide to the active metabolite R-138727.
PMID:20228231 Hagihara K et al; Drug Metab Dispos 38 (6): 898-904 (2010)
The active metabolite has an elimination half-life of about 7.4 hours (range 2-15 hours).
The radioactivity terminal elimination half-life seemed to be similar in mice and rats, approximately 24 hr, but it is considerably longer in dogs, approximately 3 days. In humans, the average terminal elimination half-life of the active metabolite R-138727 was approximately 7 hours. /R-138727/
European Medicines Agency (EMA), Evaluation of Medicines for Human Use; Assessment Report for Effient, p.12 (2009). Available from, as of October 18, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
Prasugrel is an thienopyridine and a prodrug which inhibits ADP receptors by irreversibly acting on the P2Y12 receptor on platelets. The active metabolite of prasugrel prevents binding of adenosine diphosphate (ADP) to its platelet receptor, impairing the ADP-mediated activation of the glycoprotein GPIIb/IIIa complex. Prasugrel is proposed to have a similar mechanism of action to clopidogrel.
The P2Y(12) receptor plays a crucial role in platelet aggregation and is the target of platelet aggregation inhibitors, including the thienopyridine compound prasugrel. The present study analyzed the effects of R-138727 (2-[1-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4-mercapto-3-piperidinylidene]acetic acid), the active metabolite of prasugrel, on recombinant wild-type and mutant human P2Y(12) receptors in order to identify the molecular site of action of R-138727. The function of wild-type and mutant P2Y(12) receptors stably expressed in Chinese hamster ovary cells was assessed by measuring the 2-methylthio-ADP-mediated inhibition of forskolin-stimulated cellular cAMP production. RESULTS: In cells expressing wild-type receptors, R-138727 potently inhibited receptor function with a half-maximal concentration below 1 uM. The mode of action was irreversible. The same effect of R-138727 was observed in cells expressing Cys17Ala/Cys270Ala constructs. In contrast, in cells expressing either a Cys97Ala construct or a Cys175Ala construct, R-138727 failed to inhibit the response to the agonist. When cells expressing wild-type receptors were pretreated with the P2 receptor antagonists ATP or suramin, no effect of R-138727 was observed. Similar experiments with N-acetylcysteine 10 uM showed no interference of N-acetylcysteine with R-138727. The experiments demonstrate a potent and irreversible action of R-138727 at the recombinant human P2Y(12) receptor. The data suggest that R-138727 interacts with cysteine 97 (upper portion of the predicted third transmembrane region) and cysteine 175 (second extracellular loop) of the receptor, which are likely to form a disulfide bridge in native receptors. Moreover, the data also suggest that this site of action of R-138727 is close to the ligand-binding site of the receptor. /R-138727/
PMID:18752581 Algaier I et al; J Thromb Haemost 6 (11): 1908-14 (2008)
Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
US Natl Inst Health; DailyMed. Current Medication Information for EFFIENT (prasugrel hydrochloride) tablet, film coated (September 2011). Available from, as of October 18, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Prasugrel