
1. Di Adreson F
2. Di-adreson-f
3. Diadresonf
4. Predate
5. Predonine
1. 50-24-8
2. Metacortandralone
3. Hydroretrocortine
4. Predonine
5. Delta-cortef
6. Deltacortril
7. Meticortelone
8. Deltahydrocortisone
9. Codelcortone
10. Cortalone
11. Prenolone
12. Sterane
13. Hydroretrocortin
14. Meti-derm
15. Prdl
16. Deltacortenol
17. Hydrodeltalone
18. Hydrodeltisone
19. Cotogesic
20. Decaprednil
21. Delcortol
22. Deltisilone
23. Dicortol
24. Donisolone
25. Dydeltrone
26. Erbacort
27. Erbasona
28. Estilsona
29. Fernisolone
30. Hydeltra
31. Hydeltrone
32. Lentosone
33. Paracortol
34. Paracotol
35. Precortancyl
36. Precortilon
37. Precortisyl
38. Prednelan
39. Prednicen
40. Predniliderm
41. Predonin
42. Rolisone
43. Scherisolon
44. Sterolone
45. Cordrol
46. Prednis
47. Prelone
48. Steran
49. Ulacort
50. Fernisolone P
51. Hostacortin H
52. Ultracorten H
53. Ultracortene-h
54. Delta-stab
55. Predne-dome
56. Decortin H
57. Co-hydeltra
58. Eazolin D
59. Di-adreson F
60. Delta F
61. Derpo Pd
62. 1-dehydrohydrocortisone
63. Solone
64. Delta(1)-hydrocortisone
65. Fernisolone-p
66. Delta(1)-dehydrocortisol
67. Delta-ef-cortelan
68. Dexa-cortidelt Hostacortin H
69. 1,2-dehydrohydrocortisone
70. Panafcortelone
71. Prednisolona
72. Prednisolonum
73. Prednisolonum [inn-latin]
74. Prednisolona [inn-spanish]
75. 1,4-pregnadiene-11beta,17alpha,21-triol-3,20-dione
76. (11beta)-11,17,21-trihydroxypregna-1,4-diene-3,20-dione
77. Ultracortene-hydrogen
78. Delta(1)-dehydrohydrocortisone
79. 1,4-pregnadiene-3,20-dione-11beta,17alpha,21-triol
80. 3,20-dioxo-11beta,17alpha,21-trihydroxy-1,4-pregnadiene
81. 11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione
82. K 1557
83. .delta.1-cortisol
84. Nsc-9120
85. Nsc-9900
86. .delta.1-hydrocortisone
87. 9phq9y1olm
88. (8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-3-one
89. .delta.1-dehydrocortisol
90. Chembl131
91. Chebi:8378
92. .delta.1-dehydrohydrocortisone
93. 11beta,17alpha,21-trihydroxypregna-1,4-diene-3,20-dione
94. Predniretard
95. Poly-pred
96. .delta.-cortef
97. Neo-delta-cortef
98. .delta.-stab
99. Component Of Ataraxoid
100. Cotolone
101. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-, (11b)-
102. Dsstox_cid_1184
103. Component Of K-predne-dome
104. Dsstox_rid_75996
105. Dsstox_gsid_21184
106. (1s,2r,10s,11s,14r,15s,17s)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
107. (8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
108. Delta(1)-cortisol
109. Deltasolone
110. Klismacort
111. Supercortisol
112. Delta(sup 1)-cortisol
113. Bubbli-pred
114. Delta(sup 1)-hydrocortisone
115. Delta(sup 1)-dehydrocortisol
116. Smr000718761
117. Ccris 980
118. Prednisolone [inn:ban:jan]
119. Hsdb 3385
120. Delta(sup 1)-dehydrohydrocortisone
121. Mls002638110
122. Nsc 9120
123. Pregna-1,20-dione, 11.beta.,17,21-trihydroxy-
124. Einecs 200-021-7
125. Unii-9phq9y1olm
126. Mfcd00003649
127. Predisolone Sodium Phosphate
128. Brn 1354103
129. Prednisolon
130. Preflam
131. Pregna-1,20-dione, 11,17,21-trihydroxy-, (11.beta.)-
132. Cas-50-24-8
133. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-, (11beta)-
134. Ncgc00094764-01
135. Prednisolone Powder
136. Delta-hydrocortisone
137. Prestwick_404
138. Delta-dehydrocortisol
139. Delta.1-cortisol
140. Aprednislon
141. Equisolon
142. Vetsolone
143. Delta-cortef (tn)
144. 11-beta,17,21-trihydroxypregna-1,4-diene-3,20-dione
145. T-pred (salt/mix)
146. 1,4-pregnadien-11-beta,17-alpha,21-triol-3,20-dione
147. 1,4-pregnadiene-11-beta,17-alpha,21-triol-3,20-dione
148. 1,4-pregnadiene-3,20-dione-11-beta,17-alpha,21-triol
149. Pregna-1,4-diene-3,20-dione, 11beta,17,21-trihydroxy-
150. Prednisolone, >=99%
151. Prestwick0_000274
152. Prestwick1_000274
153. Prestwick2_000274
154. Prestwick3_000274
155. Delta-dehydrohydrocortisone
156. Prednisolone [ep]
157. Prednisolone [mi]
158. (+)-prednisolone
159. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-
160. Prednisolone [inn]
161. Prednisolone [jan]
162. Ec 200-021-7
163. Prednisolone [hsdb]
164. Schembl3233
165. K-predne-dome (salt/mix)
166. Prednisolone [vandf]
167. Prednisolone Anhydrous
168. .delta.(sup 1)-cortisol
169. Bspbio_000148
170. Prednisolone [mart.]
171. (11alpha)-11,17,21-trihydroxypregna-1,4-diene-3,20-dione
172. 4-08-00-03467 (beilstein Handbook Reference)
173. Mls001304083
174. Mls002154250
175. Mls002207037
176. Mls002548883
177. Prednisolone [usp-rs]
178. Prednisolone [who-dd]
179. Prednisolone [who-ip]
180. Spbio_002367
181. Bpbio1_000164
182. Gtpl2866
183. Dtxsid9021184
184. .delta.(sup 1)-hydrocortisone
185. Bdbm19190
186. Prednisolone (jp17/usp/inn)
187. Nsc9120
188. Nsc9900
189. Prednisolone [green Book]
190. .delta.(sup 1)-dehydrocortisol
191. Hms1568h10
192. Hms2090j05
193. Hms2095h10
194. Hms2230p10
195. Hms3259e09
196. Hms3712h10
197. Prednisolone [orange Book]
198. Prednisolone [ep Monograph]
199. Prednisone Impurity B [ep]
200. Bcp09053
201. Zinc3833821
202. Prednisolone [usp Monograph]
203. Tox21_111327
204. Tox21_201673
205. Tox21_302987
206. Lmst02030179
207. Prednisolonum [who-ip Latin]
208. S1737
209. .delta.(sup 1)-dehydrohydrocortisone
210. Akos015894935
211. Tox21_111327_1
212. Ac-1773
213. Ccg-220274
214. Db00860
215. Nc00473
216. Ncgc00179649-01
217. Ncgc00179649-02
218. Ncgc00179649-03
219. Ncgc00179649-04
220. Ncgc00179649-06
221. Ncgc00256577-01
222. Ncgc00259222-01
223. As-13665
224. Hy-17463
225. Prednisolone 1000 Microg/ml In Methanol
226. Prednisolone (ema Epar: Veterinary)
227. Prednisolone 100 Microg/ml In Acetonitrile
228. P0637
229. En300-53017
230. Prednicarbate Impurity A [ep Impurity]
231. C07369
232. D00472
233. D91990
234. Hydrocortisone Impurity A [ep Impurity]
235. Prednisolone, Vetranal(tm), Analytical Standard
236. 003p649
237. A929791
238. Chloroptic-p S.o.p. Component Prednisolone
239. Methylprednisolone Impurity K [ep Impurity]
240. Sr-01000837502
241. Q-201616
242. Sr-01000837502-2
243. 11b,17,21-trihydroxypregna-1,4-diene-3,20-dione
244. Brd-k98039984-001-03-0
245. Brd-k98039984-001-06-3
246. Prednisolone Acetate Impurity B [ep Impurity]
247. Prednisolone Component Of Chloroptic-p S.o.p.
248. Q11426176
249. 11beta,17,21-trihydroxy-1,4-pregnadiene-3,20-dione
250. Delta-1-cortisol; Prednicarbate Ep Imp A; Supercortisol
251. Prednisolone, British Pharmacopoeia (bp) Assay Standard
252. Z1245633279
253. 11-.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione
254. 1,4-pregnadien-11-.beta.,17-.alpha.,21-triol-3,20-dione
255. 1,4-pregnadiene-11-.beta.,17-.alpha.,21-triol-3,20-dione
256. 1,4-pregnadiene-3,20-dione-11-.beta.,17-.alpha.,21-triol
257. Prednisolone, European Pharmacopoeia (ep) Reference Standard
258. Pregna-1,4-diene-3,20-dione, 11.beta.,17,21-trihydroxy-
259. 11,17,21-trihydroxypregna-1,4-diene-3,20-dione, (11.beta.)-
260. 11-.beta.,17-.alpha.,21-trihydroxy-1,4-pregnadiene-3,20-dione
261. 11-.beta.,17-.alpha.,21-trihydroxypregna-1,4-diene-3,20-dione
262. 11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione
263. 11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione.
264. 11.beta.,17.alpha.,21-trihydroxypregna-1,4-diene-3,20-dione
265. Prednisolone, United States Pharmacopeia (usp) Reference Standard
266. Prednisolone For Peak Identification, European Pharmacopoeia (ep) Reference Standard
267. Prednisolone For System Suitability, European Pharmacopoeia (ep) Reference Standard
268. Prednisolone, Pharmaceutical Secondary Standard; Certified Reference Material
269. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-, (11.beta)-
270. (1s,2r,10s,11s,14r,15s,17s)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadeca-3,6-dien-5-one
271. (8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-8,10,13-trimethyl-3-oxo-6,7,9,11,12,14,15,16-octahydrocyclopenta[a]phenanthrene-17-carboxylic Acid;prednisolone
272. 8056-11-9
273. Prednisolone Solution, 100 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
274. Tua
1. Hydeltra-t.b.a.
2. Prednisolone Tebutate
3. Prednisolone Trimethylacetate
4. Prednisolone Pivalate
5. Sintisone
6. Prednisolone Steaglate
7. Prednisolone Succinate
8. Prednisolone Sodium Succinate
9. Prednisolone Hemisuccinate
10. Prednisolone Hydrogen Succinate
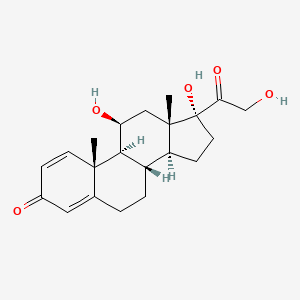
| Molecular Weight | 360.4 g/mol |
|---|---|
| Molecular Formula | C21H28O5 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 360.19367399 g/mol |
| Monoisotopic Mass | 360.19367399 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 724 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Blephamide |
| PubMed Health | Sulfacetamide/Prednisolone (Into the eye) |
| Drug Classes | Sulfonamide/Corticosteroid Combination |
| Active Ingredient | sulfacetamide sodium; Prednisolone acetate |
| Dosage Form | Suspension |
| Route | Ophthalmic |
| Strength | 0.2%; 10% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 12 | |
|---|---|
| Drug Name | Orapred |
| PubMed Health | Prednisolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppreant |
| Active Ingredient | Prednisolone sodium phosphate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 15mg base/5ml |
| Market Status | Prescription |
| Company | Concordia Pharms |
| 3 of 12 | |
|---|---|
| Drug Name | Pediapred |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppreant, Ophthalmologic Agent |
| Active Ingredient | Prednisolone sodium phosphate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 5mg base/5ml |
| Market Status | Prescription |
| Company | Seton Pharm |
| 4 of 12 | |
|---|---|
| Drug Name | Pred-g |
| Active Ingredient | prednisolone acetate; Gentamicin sulfate |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 1%; 0.6%; eq 0.3% base |
| Market Status | Prescription |
| Company | Allergan |
| 5 of 12 | |
|---|---|
| Drug Name | Prednisolone |
| Drug Label | DESCRIPTIONPrednisolone Oral Solution contains prednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Prednisolone is a... |
| Active Ingredient | Prednisolone |
| Dosage Form | Tablet; Syrup |
| Route | Oral |
| Strength | 15mg/5ml; 5mg |
| Market Status | Prescription |
| Company | Wockhardt; Alpharma; Pharm Aoc; Watson Labs; Hi Tech Pharma; Vintage |
| 6 of 12 | |
|---|---|
| Drug Name | Prelone |
| Active Ingredient | Prednisolone |
| Dosage Form | Syrup |
| Route | Oral |
| Strength | 15mg/5ml |
| Market Status | Prescription |
| Company | Teva |
| 7 of 12 | |
|---|---|
| Drug Name | Blephamide |
| PubMed Health | Sulfacetamide/Prednisolone (Into the eye) |
| Drug Classes | Sulfonamide/Corticosteroid Combination |
| Active Ingredient | sulfacetamide sodium; Prednisolone acetate |
| Dosage Form | Suspension |
| Route | Ophthalmic |
| Strength | 0.2%; 10% |
| Market Status | Prescription |
| Company | Allergan |
| 8 of 12 | |
|---|---|
| Drug Name | Orapred |
| PubMed Health | Prednisolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppreant |
| Active Ingredient | Prednisolone sodium phosphate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 15mg base/5ml |
| Market Status | Prescription |
| Company | Concordia Pharms |
| 9 of 12 | |
|---|---|
| Drug Name | Pediapred |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppreant, Ophthalmologic Agent |
| Active Ingredient | Prednisolone sodium phosphate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 5mg base/5ml |
| Market Status | Prescription |
| Company | Seton Pharm |
| 10 of 12 | |
|---|---|
| Drug Name | Pred-g |
| Active Ingredient | prednisolone acetate; Gentamicin sulfate |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 1%; 0.6%; eq 0.3% base |
| Market Status | Prescription |
| Company | Allergan |
| 11 of 12 | |
|---|---|
| Drug Name | Prednisolone |
| Drug Label | DESCRIPTIONPrednisolone Oral Solution contains prednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Prednisolone is a... |
| Active Ingredient | Prednisolone |
| Dosage Form | Tablet; Syrup |
| Route | Oral |
| Strength | 15mg/5ml; 5mg |
| Market Status | Prescription |
| Company | Wockhardt; Alpharma; Pharm Aoc; Watson Labs; Hi Tech Pharma; Vintage |
| 12 of 12 | |
|---|---|
| Drug Name | Prelone |
| Active Ingredient | Prednisolone |
| Dosage Form | Syrup |
| Route | Oral |
| Strength | 15mg/5ml |
| Market Status | Prescription |
| Company | Teva |
Anti-Inflammatory Agents, Steroidal; Antineoplastic Agents, Hormonal; Glucocorticoids, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ophthalmic corticosteroids are indicated in the treatment of corticosteroid-responsive allergic and inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. /Corticosteroids (Ophthalmic); Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 906
VET: Hormonal therapy for neoplasia commonly involves the use of glucocorticoids. Direct antitumor effects are related to their lympholytic properties; glucocorticoids can inhibit mitosis, RNA synthesis, and protein synthesis in sensitive lymphocytes. Glucocorticoids are considered cell-cycle nonspecific and are often used in chemotherapeutic protocols after induction by another agent. Prednisolone /is/ commonly used to treat lymphoreticular neoplasms in combination with other drugs. Because /it/ readily enters the CSF, ... prednisolone /is/ especially useful in treatment of leukemias and lymphomas of the CNS.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2150
Indicated in a wide range of endocrine, rheumatic, allergic, dermatologic, respiratory, hematologic, neoplastic, and other disorders.
Hussar, D.A. (ed.). Modell's Drugs in Current Use and New Drugs. 38th ed. New York, NY: Springer Publishing Co., 1992., p. 135
For more Therapeutic Uses (Complete) data for PREDNISOLONE (28 total), please visit the HSDB record page.
VET: IT OFTEN MAY BE CONTRAINDICATED IN CONGESTIVE HEART FAILURE, DIABETES OR OSTEOPOROSIS. EXCEPT FOR EMERGENCY LIFE SAVING USE, IT SHOULD BE OMITTED IN TUBERCULOSIS, CHRONIC NEPHRITIS, CUSHINGOID SYNDROMES, & PEPTIC ULCER CASES.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 479
Side effects and compliance were examined in 63 pediatric patients (ages 10 mo-14 yr) with acute asthma who received an oral dose of 1-2 mg/kg prednisolone (Solone; Panafcortelone) as a whole or crushed tablet or in liquid form for 7 days. Up to 44% of patients either refused to take or vomited the drug on the first day. Improved acceptability of prednisolone occurred with time, but prescribing practices indicated short-term treatment of 1 to 4 days was common. Abdominal pain and mood changes occurred in 19% and 80% of patients, respectively, at some stage of the study period. It was concluded that oral prednisolone is poorly tolerated in pediatric patients and its use may lead to suboptimal therapy.
Dawson KP et al; Aust J Hosp Pharm 22 (Aug): 278-82 (1992)
Glucocorticoid use in children is not only associated with the side effects which are seen in adults, but also with severe adverse effects on statural growth. As little as 2.5-5.0 mg prednisolone/day can cause a retardation in statural growth. A direct relationship exists between the dose of glucocorticoid used and statural growth. The use of knemometry, a sensitive technique for measuring the growth of long bones in children has increased the accuracy of growth rate measurements. Many factors, such as disease process, sex, daily vs alternate day therapy, ethnic variations or whether the patient has been immobilized must be considered when evaluating the effects on stature of a particular glucocorticoid.
PMID:8495277 Avioli LV; Br J Rheumatol 32 (Suppl 2): 27-30 (1993)
RESULTS FROM CONTROLLED TRIAL, INDICATE THAT PREDNISOLONE TREATMENT IS NOT BENEFICIAL & CAN BE DETRIMENTAL IN ACUTE NEUROPATHY OF UNDETERMINED ETIOLOGY.
PMID:80682 HUGHES RAC ET AL; LANCET 2 (OCT 7): 750 (1978)
For more Drug Warnings (Complete) data for PREDNISOLONE (48 total), please visit the HSDB record page.
Prednisolone is indicated to treat endocrine, rheumatic, and hematologic disorders; collagen, dermatologic, ophthalmic, respiratory, and gastrointestinal diseases; allergic and edematous states; and other conditions like tuberculous meningitis.
FDA Label
Alleviation of inflammatory and clinical parameters associated with recurrent airway obstruction (RAO) in horses, in combination with environmental control.
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Prednisolone has a short duration of action as the half life is 2.1-3.5 hours. Corticosteroids have a wide therapeutic window as patients make require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
QH02AB06
S01BA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EA - Corticosteroids acting locally
A07EA01 - Prednisolone
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA04 - Prednisolone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AA - Corticosteroids, weak (group i)
D07AA03 - Prednisolone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XA - Corticosteroids, weak, other combinations
D07XA02 - Prednisolone
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02A - Corticosteroids for systemic use, plain
H02AB - Glucocorticoids
H02AB06 - Prednisolone
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AD - Corticosteroids
R01AD02 - Prednisolone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA04 - Prednisolone
S - Sensory organs
S01 - Ophthalmologicals
S01C - Antiinflammatory agents and antiinfectives in combination
S01CB - Corticosteroids/antiinfectives/mydriatics in combination
S01CB02 - Prednisolone
S - Sensory organs
S02 - Otologicals
S02B - Corticosteroids
S02BA - Corticosteroids
S02BA03 - Prednisolone
S - Sensory organs
S03 - Ophthalmological and otological preparations
S03B - Corticosteroids
S03BA - Corticosteroids
S03BA02 - Prednisolone
Absorption
Oral prednisolone reaches a Cmax of 113-1343ng/mL with a Tmax of 1.0-2.6 hours. Oral prednisolone is approximately 70% bioavailable.
Route of Elimination
Prednisolone is over 98% eliminated in urine.
Volume of Distribution
A 0.15mg/kg dose of prednisolone has a volume of distribution of 29.3L, while a 0.30mg/kg dose has a volume of distribution of 44.2L.
Clearance
A 0.15mg/kg dose of prednisolone has a clearance of 0.09L/kg/h, while a 0.30mg/kg dose has a clearance of 0.12L/kg/h.
A randomized crossover study was conducted to compare the pharmacokinetics and pharmacodynamics of 30 mg prednisolone in a plain oral tablet (Precortisyl) with those of an enteric coated tablet (Deltacortril) in 8 patients (ages 63-81 yr) with chronic obstructive pulmonary disease and in 8 healthy males (ages 22-44 yr). Although drug absorption was considerably slower from the enteric coated tablet, peak plasma levels and total area under the concn-time curve were equivalent for the formulations. Adrenal suppression was significantly less in volunteers after enteric coated than after plain tablets. This difference was not significant in patients. Plasma cortisol levels declined more slowly after enteric coated tablets in both groups. Blood glucose levels increased over 8 hr in both groups. It was concluded that in patients with chronic obstructive pulmonary disease, peak plasma levels and total area under the concn-time curve of plain and enteric coated prednisolone tablets are equivalent; enteric coated tablets result in a lag in the decline of plasma cortisol and, in volunteers, a less marked suppression of cortisol.
PMID:1524961 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1381435 Adair CG et al; Br J Clin Pharmacol 33 (5): 495-9 (1992)
The transfer of prednisolone to breast milk was studied in 3 nursing women (ages 28-37 yr) who received an intravenous injection of 50 mg prednisolone sodium phosphate (Hydeltrasol). Concn of prednisolone in milk declined more rapidly than in serum, but were similar to expected unbound serum levels. Milk levels ranged from about 15% to 40% of serum levels. The exchange between unbound drug in serum and breast milk appeared to be relatively rapid and bidirectional. An average of 0.025% (0.01-0.49%) of the prednisolone dose was recovered in milk. It was concluded that the transfer of prednisolone to breast milk does not appear to pose a clinically significant risk.
PMID:8453851 Greenberger PA et al; Clin Pharmacol Ther 53 (3): 324-8 (1993)
The pharmacokinetics of prednisolone after oral and intravenous administration of 10 and 20 mg have been studied. Serum protein binding of prednisolone was also measured after the iv injections. The bioavailability after oral administration was 84.5% after 10 mg and 77.6% after 20 mg (p>0.05). Dose dependent pharmacokinetics were found, the VDss and Clt being significantly larger (p<0.01) after 20 mg iv than after 10 mg iv. The protein binding of prednisolone in all subjects was non-linear, and is the most likely cause of the dose dependent pharmacokinetics, as there was no dose dependent variation in elimination half-time.
PMID:6861855 Bergrem H et al; Eur J Clin Pharmacol 24 (3): 415-9 (1983)
Doses of 16, 32, 48 and 64 mg prednisolone were administered intravenously to normal volunteers who also received 100 prednisolone orally. Plasma prednisolone concentrations were estimated by quantitative thin layer chromatography. The bioavailability fraction was 1.063 +/- 0.154 (s.d.) indicating complete availability of prednisolone following oral administration. The mean T 1/2 over all doses were 4.11 +/- 0.97 (s.d.) hr and there was no evidence of a dose-related change in its value. The mean systemic clearance over all doses was 0.104 +/- 0.034 (s.d) L/hr/kg. There was no evidence of a dose-related change in clearance or in the apparent volume of distribution (overall mean 0.588 +/- 0.152 L/kg). The area under the plasma concentration-time curve was linearly related to dose. Plasma concentration-time curves normalised for dose were superimposable. It was concluded that over the dose range investigated, non-linear pharmacokinetic behavior had not been demonstrated in this group of normal volunteers.
PMID:7437263 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430141 Al-Habet S, Rogers HJ; Br J Clin Pharmacol 10 (5): 503-8 (1980)
For more Absorption, Distribution and Excretion (Complete) data for PREDNISOLONE (13 total), please visit the HSDB record page.
Prednisolone can be reversibly metabolized to [prednisone] which is then metabolized to 17,21-dihydroxy-pregnan-1,4,6-trien-3,11,30-trione (M-XVII), 20-dihydro-prednisone (M-V), 6hydroxy-prednisone (M-XII), 6-hydroxy-prednisone (M-XIII), or 20-dihydro-prednisone (M-IV). 20-dihydro-prednisone is metabolized to 17,20,21-trihydroxy-5-pregn-1-en-3,11-dione(M-XVIII). Prednisolone is metabolized to 6-prednisolone (M-XI), 20-dihydro-prednisolone (M-III), 20-dihydro-prednisolone (M-II), 6hydroxy-prednisolone (M-VII), or 6hydroxy-prednisolone(M-VI). 6hydroxy-prednisolone is metabolized to 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-X). 6hydroxy-prednisolone is metabolized to 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-VIII), 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-IX), and 6,11,17,21-tetrahydroxy-5-pregn-1-en-3,20-dione (M-XIV). MVIII is metabolized to 6,11,17,20,21-pentahydroxy-5-pregn-1-en-3-one (M-XV) and then to MXIV, while MIX is metabolized to 6,11,17,20,21-pentahydroxy-5-pregn-1-en-3-one (M-XVI) and then to MXIV. These metabolites and their glucuronide conjugates are excreted predominantly in the urine.
Reduction of the 4,5 double bond can occur at both hepatic and extrahepatic sites and yields an inactive substance. Subsequent reduction of the 3-ketone substituent to a 3-hydroxyl to form tetrahydrocortisol has been demonstrated only in liver. Most of the ring a - reduced metabolites are enzymatically coupled through the 3-hydroxyl with sulfate or with glucuronic acid to form water soluble sulfate esters or glucuronides, and they are excreted as such.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1446
Conjugated mostly in liver but also in kidney. /Human, oral/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1963
In the present study the metabolism of prednisolone in the isolated, perfused, dual recirculating human placental lobule was reexamined, using a perfusate based on tissue culture medium 199. Four metabolites were identified in both the maternal and fetal compartments in 6 hr perfusions by comparison of relative retention times measured by HPLC and capillary GC and of mass spectra recorded by capillary GC/MS with those of authentic reference standards. The steroids were derivatized as the MO-TMS ethers for mass spectral measurements. Analysis of samples from five perfusion experiments resulted in the following percentage conversions after 6 hr perfusion (mean + or - standard deviation, maternal and fetal perfusate, respectively): prednisone (49.1 + or - 7.8, 49.1 + or - 6.6), 20 alpha-dihydroprednisone (0.84 + or - 0.29, 0.81 + or - 0.35), 20 beta-dihydroprednisone (39.1 + or - 6.7, 39.2 + or - 5.9), 20 beta-dihydroprednisolone (6.8 + or - 2.7, 6.3 + or - 1.6) and unmetabolized prednisolone (4.1 + or - 1.8, 4.6 + or - 2.1). No evidence was found for metabolites formed by 6 beta-hydroxylation or cleavage of the C17-C20 bond.
PMID:2069869 Addison RS et al; J Steroid Biochem Mol Biol 39 (1): 83-90 (1991)
A randomized, four-way cross-over study was conducted in eight healthy male volunteers to determine the relative and absolute bioavailability of prednisone (PN) and prednisolone (PL). PN and PL were administered as single, oral 10-mg tablet doses and as 10-mg zero-order 0.5-hour intravenous infusions. Comparable mean PN and PL maximum plasma concentrations (Cmax), times for Cmax, areas under the plasma concentration-time curves (AUC), and apparent elimination rate constants between tablet treatments demonstrated that PN and PL tablets were bioequivalent. Absolute bioavailability (F) determinations based on plasma PL concentrations were independent of which IV treatment was used as reference and indicated complete systemic availability of PL from both PN and PL tablets. However, F based on plasma PN data was contradictory. Using IV PN as reference, approximately 70% systemic availability was observed from both tablets, whereas using IV PL as reference, systemic availability was greater than unity. PN and PL are model compounds that exemplify the difficulties involved in accurately determining the relative and absolute bioavailability of substances that undergo reversible metabolism.
PMID:3350994 Ferry JJ et al; J Clin Pharmacol 28(1):81-7 (1988)
Prednisone, prednisolone, and methylprednisolone are currently administered in association with cyclosporin A in the postoperative treatment of transplant patients. The aim of this work was to evaluate the effects of these corticosteroids on the expression of several forms of cytochromes p450, including p450 1A2, 2D6, 2E1, and 3A, and on cyclosporin A oxidase activity in human liver. For this purpose, human hepatocytes prepared from lobectomies were maintained in culture in a serum-free medium, in collagen-coated dishes, for 96-144 hr, in the absence or presence of 50-100 uM corticosteroids, rifampicin, or dexamethasone. To mimic more closely the current clinical protocol, hepatocyte cultures were also co-treated with corticosteroids and cyclosporin A or ketoconazole (a selective inhibitor of cytochromes p450 3A). Cyclosporin A oxidase activity, intracellular retention of cyclosporin A oxidized metabolites within hepatocytes, accumulation of cytochromes p450 proteins and corresponding messages, and de novo synthesis and half-lives of these cytochromes p450 were measured in parallel in these cultures. Our results, obtained from seven different hepatocyte cultures, showed that 1) dexamethasone and prednisone, but not prednisolone or methylprednisolone, were inducers of cytochrome p450 3A, at the level of protein and mRNA accumulation, as well as of cyclosporin A oxidase activity, known to be predominantly catalyzed by these cytochromes p450; 2) although corticosteroids are known to be metabolized in human liver, notably by cytochrome p450 3A, partial or total inhibition of this cytochromes p450 by cyclosporin or ketoconazole, respectively, did not affect the inducing efficiency of these molecules; 3) corticosteroids did not affect the half-life of cytochrome p450 3A or the accumulation of other forms of cytochromes p450, including 1A2, 2D6, and 2E1; 4) chronic treatment of cells with cyclosporin did not affect cytochrome p450 3A accumulation; 5) corticosteroids were all competitive inhibitors of cyclosporin A oxidase in human liver microsomes, with Ki values of 61 + or - 12, 125 + or - 25, 190 + or - 38, and 210 + or - 42 uM for dexamethasone, prednisolone, prednisone, and methylprednisolone, respectively; and 6) chronic treatment of cells with corticosteroids did not influence the excretion of oxidized metabolites of cyclosporin from the cells.
PMID:1614409 Pichard L et al; Mol Pharmacol 41 (6): 1047-55 (1992)
Prednisolone has a plasma half life of 2.1-3.5 hours. This half life is shorter in children and longer in those with liver disease.
...Prednisolone (60 mg/sq m/day in three divided doses) was administered both orally and intravenously /to 23 children with acute lymphoblastic leukemia (ALL) (aged 2-15 years)/, and samples were obtained on several days during the initial 5 weeks of remission induction therapy. ...The median unbound clearance (32 L/hr/sq m) was lower, and the half-life (3.6 hr) longer than previously reported in childhood ALL.
PMID:12698270 Petersen KB et al; Cancer Chemother Pharmacol 51 (6): 465-73 (2003)
Doses of 16, 32, 48 and 64 mg prednisolone were administered intravenously to normal volunteers who also received 100 prednisolone orally. ...The mean T 1/2 over all doses were 4.11 +/- 0.97 (s.d.) hr and there was no evidence of a dose-related change in its value.
PMID:7437263 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430141 Al-Habet S, Rogers HJ; Br J Clin Pharmacol 10 (5): 503-8 (1980)
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
Although altered homeostatic regulation, including disturbance of 24-h rhythms, is often observed in the patients undergoing glucocorticoid therapy, the mechanisms underlying the disturbance remains poorly understood. We report here that chronic treatment with a synthetic glucocorticoid, prednisolone, can cause alteration of circadian clock function at molecular level. Treatment of cultured hepatic cells (HepG2) with prednisolone induced expression of Period1 (Per1), and the prednisolone treatment also attenuated the serum-induced oscillations in the expression of Period2 (Per2), Rev-erbalpha, and Bmal1 mRNA in HepG2 cells. Because the attenuation of clock gene oscillations was blocked by pretreating the cells with a Per1 antisense phosphothioate oligodeoxynucleotide, the extensive expression of Per1 induced by prednisolone may have resulted in the reduced amplitude of other clock gene oscillations. Continuous administration of prednisolone into mice constitutively increased the Per1 mRNA levels in liver and skeletal muscle, which seems to attenuate the oscillation in the expressions of Per2, Rev-erbalpha, and Bmal1. However, a single daily administration of prednisolone at the time of day corresponding to acrophase of endogenous glucocorticoid levels had little effect on the rhythmic expression of clock genes. These results suggest a possible pharmacological action by prednisolone on the core circadian oscillation mechanism and indicate the possibility that the alteration of clock function induced by prednisolone can be avoided by optimizing the dosing schedule.
PMID:16269518 Koyanagi S et al; Mol Endocrinol 20 (3): 573-83 (2006)
Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2128