


1. Aic316
2. Bay 57-1293
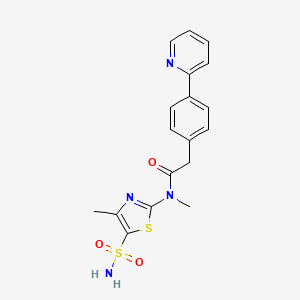
3. N-(5-(aminiosulfonyl)-4-methyl-1,3-thiazol-2-yl)-n-methyl-2-(4-(2-pyridinyl)phenyl)acetamide
1. 348086-71-5
2. Bay 57-1293
3. Bay-57-1293
4. Aic316
5. Pritelivir [inn]
6. N-methyl-n-(4-methyl-5-sulfamoylthiazol-2-yl)-2-(4-(pyridin-2-yl)phenyl)acetamide
7. Bay57-1293
8. Aic-316
9. N-methyl-n-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-(4-pyridin-2-ylphenyl)acetamide
10. 07hq1tj4je
11. Pritelivir (bay 57-1293)
12. 348086-71-5 (free Base)
13. Bay 57-1293;aic316
14. Unii-07hq1tj4je
15. Benzeneacetamide, N-(5-(aminosulfonyl)-4-methyl-2-thiazolyl)-n-methyl-4-(2-pyridinyl)-
16. N-(5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl)-n-methyl-2-(4-(2-pyridinyl)phenyl)acetamide
17. N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-n-methyl-2-[4-(2-pyridinyl)phenyl]acetamide
18. N-methyl-n-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-(4-(pyridin-2-yl)phenyl)acetamide
19. N-methyl-n-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-[4-(pyridin-2-yl)phenyl]acetamide
20. Pritelivir;aic316
21. Pritelivir [who-dd]
22. Pritelivirbay-57-1293
23. Schembl1074614
24. Pritelivir(bay 57-1293)
25. Chembl4069597
26. Aic 316
27. Dtxsid70188344
28. Ex-a247
29. Hms3653b15
30. Hms3744e03
31. Bcp08742
32. Yna08671
33. Zinc3955689
34. Mfcd18633192
35. S7546
36. Akos026750515
37. Ccg-268675
38. Cs-1693
39. Db11844
40. Ks-5353
41. Sb19731
42. Ncgc00378984-02
43. Ac-33065
44. Da-42669
45. Hy-15303
46. Ls-14637
47. N-(5-(aminiosulfonyl)-4-methyl-1,3-thiazol-2-yl)-n-methyl-2-(4-(2-pyridinyl)phenyl)acetamide
48. Ft-0732285
49. Sw219861-1
50. A13324
51. A857450
52. Q15410303
53. N-methyl-n-(4-methyl-5-sulfamoyl-thiazol-2-yl)-2-[4-(2-pyridyl)phenyl]acetamide
54. Bay-571293; Aic-316; Aic 316;n-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-n-methyl-2-[4-(2-pyridinyl)phenyl]acetamide;n-methyl-n-(4-methyl-5-sulfamoylthiazol-2-yl)-2-(4-(pyridin-2-yl)phenyl)acetamide
55. N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-n-methyl-2-[4-(2-pyridinyl)phenyl] Acetamide
56. N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-n-methyl-2-[4-(2-pyridyl)phenyl]acetamide
57. N-[5-(aminosulphonyl)-4-methyl-1,3-thiazol-2-yl]-n-methyl-2-[4-(2-pyridinyl)phenyl]acetamide
| Molecular Weight | 402.5 g/mol |
|---|---|
| Molecular Formula | C18H18N4O3S2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 402.08203280 g/mol |
| Monoisotopic Mass | 402.08203280 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 617 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
