
1. Compazine
2. Edisylate Salt, Prochlorperazine
3. Edisylate, Prochlorperazine
4. Maleate, Prochlorperazine
5. Prochlorperazine Edisylate
6. Prochlorperazine Edisylate Salt
7. Prochlorperazine Maleate
8. Salt, Prochlorperazine Edisylate
1. 58-38-8
2. Prochlorperazin
3. Compazine
4. Prochlorpromazine
5. Chlormeprazine
6. Chlorperazine
7. Procloperazine
8. Capazine
9. Prochlorpemazine
10. Proclorperazine
11. Meterazin
12. Meterazine
13. Stemetil
14. Tementil
15. Kronocin
16. Compro
17. Emelent
18. Nipodal
19. Temetid
20. Prochlorpermazine
21. Proclorperazina
22. Bayer A 173
23. Prochlorperazine Maleate
24. Prochloroperazine
25. Prochlorperazinum
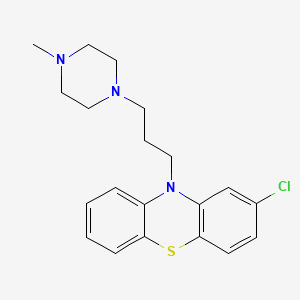
26. 2-chloro-10-[3-(4-methylpiperazin-1-yl)propyl]phenothiazine
27. 2-chloro-10-(3-(4-methyl-1-piperazinyl)propyl)phenothiazine
28. Vertigon
29. 2-chloro-10-[3-(4-methylpiperazin-1-yl)propyl]-10h-phenothiazine
30. Skf 4657
31. 6140 Rp
32. Rp 6140
33. 3-chloro-10-(3-(1-methyl-4-piperazinyl)propyl)phenothiazine
34. 10h-phenothiazine, 2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]-
35. Buccastem
36. Chloperazine
37. 2-chloro-10-(3-(1-methyl-4-piperazinyl)propyl)-phenothiazine
38. 3-chloro-10-(3-(4-methyl-1-piperazinyl)propyl)phenothiazine
39. Mls000028600
40. N-(gamma-(4'-methylpiperazinyl-1')propyl)-3-chlorophenothiazine
41. 2-chloro-10-(3-(4-methylpiperazin-1-yl)propyl)-10h-phenothiazine
42. 10h-phenothiazine, 2-chloro-10-(3-(4-methyl-1-piperazinyl)propyl)-
43. Chloro-3 (n-methylpiperazinyl-3 Propyl)-10 Phenothiazine
44. Yhp6ylt61t
45. Prochlorperazine Base
46. Smr000058705
47. Prochlorperazine Mesylate
48. Chebi:8435
49. Compazine Suppositories
50. Phenothiazine, 2-chloro-10-(3-(4-methyl-1-piperazinyl)propyl)-
51. Prochlorperazinum [inn-latin]
52. Proclorperazina [inn-spanish]
53. Proazine
54. Mls001148133
55. Compro (tn)
56. Nsc17478
57. Nsc167375
58. Hsdb 3171
59. Cas-84-02-6
60. Compazine (*maleate*)
61. Smr000653454
62. Einecs 200-379-4
63. Unii-yhp6ylt61t
64. Chloropernazine
65. Phenothiazine, 2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]-
66. Prochlorperazine (jan/usp/inn)
67. 2-chloro-10-(3-(4-methyl-1-piperazinyl)propyl)-10h-phenothiazine
68. Chloro-3 (n-methylpiperazinyl-3 Propyl)-10 Phenothiazine [french]
69. Prochlorperazine [usp:inn:ban:jan]
70. Eskatrol (salt/mix)
71. Spectrum_000840
72. Opera_id_244
73. Prestwick0_000399
74. Prestwick1_000399
75. Prestwick2_000399
76. Prestwick3_000399
77. Spectrum2_001297
78. Spectrum3_000881
79. Spectrum4_000972
80. Spectrum5_001339
81. Lopac-p-9178
82. Chembl728
83. Probes1_000265
84. Probes2_000307
85. Lopac0_001034
86. Schembl18429
87. Bspbio_000617
88. Bspbio_002394
89. Kbiogr_001343
90. Kbiogr_002304
91. Kbioss_001320
92. Kbioss_002306
93. Prochlorperazine [mi]
94. Cid_91499
95. Mls006011830
96. Divk1c_000413
97. Prochlorperazine [inn]
98. Prochlorperazine [jan]
99. Spbio_001333
100. Spbio_002538
101. Prochlorperazine [hsdb]
102. Bpbio1_000679
103. Gtpl7279
104. Prochlorperazine [vandf]
105. Dtxsid7023514
106. Prochlorperazine [mart.]
107. Bdbm78434
108. Kbio1_000413
109. Kbio2_001320
110. Kbio2_002304
111. Kbio2_003888
112. Kbio2_004872
113. Kbio2_006456
114. Kbio2_007440
115. Kbio3_001762
116. Kbio3_002784
117. Prochlorperazine [who-dd]
118. Cmap_000013
119. Ninds_000413
120. Hms2231p21
121. Stl371212
122. Zinc19796018
123. Prochlorperazine [orange Book]
124. Akos003600762
125. Ccg-205112
126. Db00433
127. Rp-6140
128. Sdccgsbi-0051005.p005
129. Idi1_000413
130. Mrf-0000068
131. Prochlorperazine [usp Monograph]
132. Ncgc00015856-01
133. Ncgc00015856-02
134. Ncgc00015856-03
135. Ncgc00015856-04
136. Ncgc00015856-05
137. Ncgc00015856-06
138. Ncgc00015856-07
139. Ncgc00015856-16
140. Ncgc00023036-03
141. Nci60_022783
142. P77
143. Sbi-0051005.p004
144. 6140 R.p.
145. Db-053199
146. Ab00053532
147. Ft-0603243
148. C07403
149. C16030
150. D00493
151. Ab00053532_29
152. Ab00053532_30
153. 058p388
154. A831861
155. L001030
156. Q2359690
157. Brd-k19352500-070-02-5
158. Brd-k19352500-070-05-8
159. Brd-k19352500-332-03-7
160. Sr-01000000260-11
161. 2-chloro-10-(3-(1-methyl-4-piperazinyl)-propyl)-phenothiazine
162. N-(.gamma.-(4'-methylpiperazinyl-1')propyl)-3-chlorophenothiazine
163. Phenothiazine, 2-chloro-10-[3-(1-methyl-4-piperazinyl)propyl]-
164. 10h-phenothiazine, 2-chloro-10-(3-(4-methyl-1-piperazinyl)propyl
165. 2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]-10h-phenothiazine #
166. 2-chloranyl-10-[3-(4-methylpiperazin-1-yl)propyl]phenothiazine;ethane-1,2-disulfonic Acid
167. 2-chloro-10-[3-(4-methyl-1-piperazinyl)propyl]phenothiazine;ethane-1,2-disulfonic Acid
168. 2-chloro-10-[3-(4-methylpiperazino)propyl]phenothiazine;ethane-1,2-disulfonic Acid
| Molecular Weight | 373.9 g/mol |
|---|---|
| Molecular Formula | C20H24ClN3S |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 373.1379466 g/mol |
| Monoisotopic Mass | 373.1379466 g/mol |
| Topological Polar Surface Area | 35 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 429 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Compro |
| PubMed Health | Prochlorperazine |
| Drug Classes | Antiemetic, Antipsychotic |
| Drug Label | Prochlorperazine is a clear, pale yellow, viscous liquid. It is sensitive to light, very slightly soluble in water, freely soluble in alcohol, in chloroform, and in ether.Each suppository, for rectal administration, contains 25 mg of prochlorperazine... |
| Active Ingredient | Prochlorperazine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Paddock |
| 2 of 6 | |
|---|---|
| Drug Name | Prochlorperazine |
| Drug Label | Prochlorperazine edisylate, 2-Chloro-10-[3-(4-methyl-1-piperazinyl)propyl]phenothiazine 1,2-ethanedisulfonate (1:1), has the following structural formula:C20H24ClN3SC2H6O6S2 MW 564.14Prochlorperazine Edis... |
| Active Ingredient | Prochlorperazine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 25mg |
| Market Status | Prescription |
| Company | G And W Labs |
| 3 of 6 | |
|---|---|
| Drug Name | Prochlorperazine maleate |
| Drug Label | Prochlorperazine is a phenothiazine derivative, present in prochlorperazine tablets as the maleate. Prochlorperazine maleate is designated chemically as 2-chloro-10-[3-(4-methyl-1- piperazinyl)propyl] phenothiazine maleate and has the following struc... |
| Active Ingredient | Prochlorperazine maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Sandoz; Teva Pharms; Mylan |
| 4 of 6 | |
|---|---|
| Drug Name | Compro |
| PubMed Health | Prochlorperazine |
| Drug Classes | Antiemetic, Antipsychotic |
| Drug Label | Prochlorperazine is a clear, pale yellow, viscous liquid. It is sensitive to light, very slightly soluble in water, freely soluble in alcohol, in chloroform, and in ether.Each suppository, for rectal administration, contains 25 mg of prochlorperazine... |
| Active Ingredient | Prochlorperazine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Paddock |
| 5 of 6 | |
|---|---|
| Drug Name | Prochlorperazine |
| Drug Label | Prochlorperazine edisylate, 2-Chloro-10-[3-(4-methyl-1-piperazinyl)propyl]phenothiazine 1,2-ethanedisulfonate (1:1), has the following structural formula:C20H24ClN3SC2H6O6S2 MW 564.14Prochlorperazine Edis... |
| Active Ingredient | Prochlorperazine |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 25mg |
| Market Status | Prescription |
| Company | G And W Labs |
| 6 of 6 | |
|---|---|
| Drug Name | Prochlorperazine maleate |
| Drug Label | Prochlorperazine is a phenothiazine derivative, present in prochlorperazine tablets as the maleate. Prochlorperazine maleate is designated chemically as 2-chloro-10-[3-(4-methyl-1- piperazinyl)propyl] phenothiazine maleate and has the following struc... |
| Active Ingredient | Prochlorperazine maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Sandoz; Teva Pharms; Mylan |
Antiemetics; Antipsychotic Agents, Phenothiazine; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
/Prochlorperazine is indicated/ for control of severe nausea and vomiting. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate ) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
/Prochlorperazine is indicated/ for the treatment of schizophrenia. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
Prochlorperazine is effective for the short-term treatment of generalized non-psychotic anxiety. However, prochlorperazine is not the first drug to be used in therapy for most patients with non-psychotic anxiety, because certain risks associated with its use are not shared by common alternative treatments (eg, benzodiazepines). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
For more Therapeutic Uses (Complete) data for Prochlorperazine (7 total), please visit the HSDB record page.
/BOXED WARNING/ Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10 week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Prochlorperazine maleate is not approved for the treatment of patients with dementia-related psychosis.
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
VET: Epinephrine may further lower, rather than elevate, blood pressures in animals on this phenothiazine derivatives.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 486
Do not use in patients with known hypersensitivity to phenothiazines.
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.).
US Natl Inst Health; DailyMed. Current Medication Information for Prochlorperazine (prochlorperazine maleate) tablet (September 2009). Available from, as of June 20, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15790
For more Drug Warnings (Complete) data for Prochlorperazine (53 total), please visit the HSDB record page.
Toxic dose: 100 ug/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 422
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT (OR LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-222
Indicated for the symptomatic treatment of severe nausea and vomiting. Indicated for the management of manifestations of psychotic disorders, such as schizophrenia and generalized non-psychotic anxiety. The use of prochlorperazine for the management of generalized non-psychotic anxiety is typically not a first-line therapy and should be limited to doses of less than 20 mg per day or for shorter than 12 weeks. Off-label uses include use in emergency settings for adult and pediatric migraines. The American Headache Society recommends the use of prochlorperazine as the first-line medication in this setting. In pediatric migraines, a non-steroidal anti-inflammatory agent is often used in combination with dopamine antagonist.
FDA Label
Prochlorperazine is an antipsychotic agent that works to promote postsynaptic inhibition of dopaminergic neurons. It also exerts its anti-emetic actions via anti-dopaminergic effects, where it displays similar efficacy as ondansteron, a 5HT-3 receptor antagonist and anti-emetic, in preventing delayed nausea and vomiting. Prochlorperazine was shown to inhibit histaminergic, cholinergic and alpha-1 adrenergic receptors. The blockade of alpha-1 adrenergic receptors may result in sedation, muscle relaxation, and hypotension. It displays anti-anxiety effects as well. Compared to other phenothiazine derivatives, prochlorperazine is less sedating and has a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. Other than its primary action on D2 receptors, one study showed that prochlorperazine may inhibit the P2X7 receptor in human macrophages, leading to inhibition of calcium ion influx.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AB - Phenothiazines with piperazine structure
N05AB04 - Prochlorperazine
Absorption
Following oral administration, prochlorperazine is reported to be well absorbed from the gastrointestinal tract. The onset of pharmacological action is about 30 to 40 minutes following oral administration and 10 to 20 minutes following intramuscular administration. The duration of action for all routes is about 3 to 4 hours. Following oral administration in healthy volunteers, the mean oral bioavailability was about 12.5%. In these patients, the time to reach the peak plasma concentrations was about 5 hours. Repeated oral dosing resulted in an accumulation of prochlorperazine and its metabolite. Following multiple twice daily dosing, the steady state of prochlorperazine was reached by 7 days.
Route of Elimination
Prochlorperazine is reported to be mainly excreted via the feces and bile. Low quantities of unchanged prochlorperazine and its metabolite were detectable in the urine.
Volume of Distribution
In a preliminary pharmacokinetic study involving healthy volunteers, the mean apparent volume of distribution following intravenous administration of 6.25 mg and 12.5 mg prochlorperazine were approximately 1401 L and 1548 L, respectively. Prochlorperazine is reported to be distributed to most body tissues with high concentrations being distributed into liver and spleen. There is evidence that phenothiazines are excreted in the breast milk of nursing mothers.
Clearance
The mean plasma clearance (CL) of prochlorperazine following intravenous administration in healthy volunteers was approximately 0.98L/h x kg. The mean renal clearance was about 23.6 mL/h.
Phenothiazines are generally well absorbed from the GI tract and from parenteral sites; however, absorption may be erratic, particularly following oral administration. Considerable interindividual variations in peak plasma concentrations have been reported. The variability may result from genetic differences in the rate of metabolism, biodegradation of the drug in the GI lumen, and/or metabolism of the drug during absorption (in the GI mucosa) and first pass through the liver.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Phenothiazines are highly bound to plasma proteins.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Phenothiazines and their metabolites are distributed into most body tissues and fluids, with high concentrations being distributed into the brain, lungs, liver, kidneys, and spleen. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Phenothiazines readily cross the placenta. It is not known if the drugs are distributed into milk; however, the size of the molecules and their ability to readily cross the blood-brain barrier suggest that the drugs would be distributed into milk.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
For more Absorption, Distribution and Excretion (Complete) data for Prochlorperazine (12 total), please visit the HSDB record page.
Prochlorperazine undergoes hepatic metabolism involving oxidation, hydroxylation, demethylation, sulfoxide formation and conjugation with glucuronic acid. The oxidation reaction is mediated by CYP2D6. N-desmethyl prochlorperazine was detected in the plasma, as well as prochlorperazine sulfoxide, prochlorperazine 7-hydroxide and prochlorperazine sulfoxide 4'-N-oxide, following oral and buccal administration. Prochlorperazine may enter the enterohepatic circulation.
Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Metabolized primarily in liver /by/ oxidation, hydroxylation, demethylation, sulfoxide formation and conjugation with glucuronic acid; metabolic alterations in side chain may also occur.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1970
After chronic administration of piperazine-substituted phenothiazine drugs ... to rats, tissues contained drug metabolites, in which piperazine ring fission by multiple oxidative n-dealkylation had occurred to give substituted ethylenediamine. Thus, n-[gamma-(2-chlorphenothiazinyl-10)-propyl]ethylenediamine ... from prochlorperazine ...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 255
Yields 2-chloro-10-(3-(4-methylpiperazin-1-yl)propyl)phenothiazine-n-oxide and 2-chloro-10-(3-(4-methylpiperazin-1-yl)propyl)phenothiazine sulfoxide in rats
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-33
For more Metabolism/Metabolites (Complete) data for Prochlorperazine (7 total), please visit the HSDB record page.
Following intravenous and single oral dose administration, the terminal elimination half live were 9 and 8 hours, respectively.
The mechanism of action of prochlorperazine has not been fully determined, but may be primarily related to its anti-dopaminergic effects. Prochlorperazine blocks the D2 dopamine receptors in the brain, which are somatodendritic autoreceptors. Inhibition of D2 receptor signaling results in the blockade of postsynaptic dopamine receptors in the mesolimbic system and an increased dopamine turnover. Nausea and vomiting are proposed to arise from peripheral or central stimulation of serotonin type 3 (5-HT3) and dopamine type 2 receptors, the predominant receptors expressed at the chemoreceptor trigger zone (CTZ). Prochlorperazine exerts antiemetic effects and was shown to inhibit apomorphine-induced vomiting by blocking D2 dopamine receptors in the CTZ..
The principal pharmacologic effects of prochlorperazine are similar to those of chlorpromazine. Prochlorperazine has weak anticholinergic effects, moderate sedative effects, and strong extrapyramidal effects. Prochlorperazine has strong antiemetic activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2518
The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2510
In the CNS, phenothiazines act principally at the subcortical levels of the reticular formation, limbic system, and hypothalamus. Phenothiazines generally do not produce substantial cortical depression; however, there is minimal information on the specific effects of phenothiazines at the cortical level. Phenothiazines also act in the basal ganglia, exhibiting extrapyramidal effects. The precise mechanism(s) of action, including antipsychotic action, of phenothiazines has not been determined, but may be principally related to antidopaminergic effects of the drugs. There is evidence to indicate that phenothiazines antagonize dopamine-mediated neurotransmission at the synapses. There is also some evidence that phenothiazines may block postsynaptic dopamine receptor sites. However, it has not been determined whether the antipsychotic effect of the drugs is causally related to their antidopaminergic effects. Phenothiazines also have peripheral and/or central antagonistic activity against alpha-adrenergic, serotonergic, histaminic (H1-receptors), and muscarinic receptors. Phenothiazines also have some adrenergic activity, since they block the reuptake of monoamines at the presynaptic neuronal membrane, which tends to enhance neurotransmission. The effects of phenothiazines on the autonomic nervous system are complex and unpredictable because the drugs exhibit varying degrees of alpha-adrenergic blocking, muscarinic blocking, and adrenergic activity. The antipsychotic activity of phenothiazines may be related to any or all of these effects, but it has been suggested that the drugs' effects on dopamine are probably most important. It has also been suggested that effects of phenothiazines on other amines (eg, gamma-aminobutyric acid [GABA]) or peptides (eg, substance P, endorphins) may contribute to their antipsychotic effect. Further study is needed to determine the role of central neuronal receptor antagonism and of effects on biochemical mediators in the antipsychotic action of the phenothiazines and other antipsychotic agents. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2510
Although the exact mechanism(s) of action has not been conclusively determined, phenothiazines have an antiemetic effect. The antiemetic activity may be mediated via a direct effect of the drugs on the medullary chemoreceptor trigger zone (CTZ), apparently by blocking dopamine receptors in the CTZ. Phenothiazines inhibit the central and peripheral effects of apomorphine and ergot alkaloids. Phenothiazines generally do not inhibit emesis caused by the action of drugs at the nodose ganglion or by local action on the GI tract. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
For more Mechanism of Action (Complete) data for Prochlorperazine (15 total), please visit the HSDB record page.