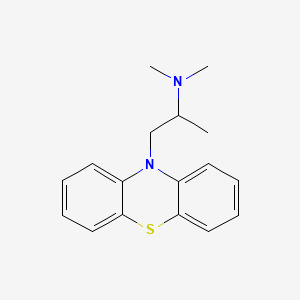

1. Atosil
2. Diphergan
3. Diprazin
4. Hydrochloride, Promethazine
5. Isopromethazine
6. Phenargan
7. Phenergan
8. Phensedyl
9. Pipolfen
10. Pipolphen
11. Proazamine
12. Promet
13. Prometazin
14. Promethazine Hydrochloride
15. Prothazin
16. Pyrethia
17. Remsed
18. Rumergan
1. Proazamine
2. 60-87-7
3. Diphergan
4. Protazine
5. Prometazin
6. Promethazin
7. Prothazin
8. Vallergine
9. Dimapp
10. Fargan
11. Procit
12. Phenargan
13. Phensedyl
14. Promazinamide
15. Promezathine
16. Isophenergan
17. Diprazine
18. Diprozin
19. Fenetazina
20. Histargan
21. Provigan
22. Thiergan
23. Fenazil
24. Hiberna
25. Phargan
26. Prorex
27. Tanidil
28. Pyrethiazine
29. Avomine
30. Lilly 1516
31. Prometazina
32. Promethacon
33. Promethegan
34. Promethazinum
35. Promethiazine
36. Iergigan
37. Lercigan
38. Pilpophen
39. Proazaimine
40. Prometasin
41. Pyrethia
42. Lilly 01516
43. N-(2'-dimethylamino-2'-methyl)ethylphenothiazine
44. Skf 1498
45. (2-dimethylamino-2-methyl)ethyl-n-dibenzoparathiazine
46. Wy 509
47. 10-[2-(dimethylamino)propyl]phenothiazine
48. Rp 3277
49. Lergigan
50. Romergan
51. 10-(2-dimethylaminopropyl)phenothiazine
52. Dimethylamino-isopropyl-phenthiazin
53. N,n,alpha-trimethyl-10h-phenothiazine-10-ethanamine
54. N-dimethylamino-2-methylethyl Thiodiphenylamine
55. A-91033
56. Nci-c60673
57. 10-(2-(dimethylamino)-2-methylethyl)phenothiazine
58. 10h-phenothiazine-10-ethanamine, N,n,alpha-trimethyl-
59. 3277 Rp
60. (+/-)-promethazine
61. 10-(2-(dimethylamino)propyl)phenothiazine
62. Phenothiazine, 10-(2-dimethylaminopropyl)-
63. N,n-dimethyl-1-(10h-phenothiazin-10-yl)propan-2-amine
64. Nsc-30321
65. Chembl643
66. 60-87-7 (free Base)
67. 10h-phenothiazine-10-ethanamine, N,n,.alpha.-trimethyl-
68. Chebi:8461
69. Ff28ejq494
70. Antiallersin
71. Fenetazine
72. Phenerzine
73. Pipolphene
74. Camergan
75. Metaryl
76. Pelpica
77. Pilothia
78. Promacot
79. Promergan
80. Promesan
81. Prometh
82. Promethazinehcl
83. Phenothiazine, 10-[2-(dimethylamino)propyl]-
84. Phenoject-50
85. Dimethyl[1-(10h-phenothiazin-10-yl)propan-2-yl]amine
86. Rp-3277
87. Ncgc00015817-05
88. Prometazine
89. Prothazine
90. Valergine
91. Dsstox_cid_3518
92. Dsstox_rid_77061
93. Dsstox_gsid_23518
94. Pro-50
95. Promethazine [inn:ban]
96. Prometazina [inn-spanish]
97. Promethazinum [inn-latin]
98. Cas-60-87-7
99. Ccris 7056
100. Hsdb 3173
101. Dimethylamino-isopropyl-phenthiazin [german]
102. Einecs 200-489-2
103. Promethazine (jan/inn)
104. Nsc 30321
105. Phenothiazine, 10-(2-(dimethylamino)propyl)-
106. N,n-dimethyl-1-phenothiazin-10-ylpropan-2-amine
107. Brn 0088554
108. Unii-ff28ejq494
109. Sominex
110. Phenergan Base
111. Sr-01000002993
112. 38878-40-9
113. Remsed (salt/mix)
114. 3389 R.p.
115. Pyrethia (salt/mix)
116. Pipolphen (salt/mix)
117. Spectrum_000868
118. Prestwick0_000888
119. Prestwick1_000888
120. Prestwick2_000888
121. Prestwick3_000888
122. Spectrum2_000840
123. Spectrum3_001019
124. Spectrum4_001149
125. Spectrum5_000977
126. Promethazine [mi]
127. (.+/-.)-promethazine
128. 10h-phenothiazine-10-ethanamine, N,n,alpha-trimethyl-, Radical Ion(1+)
129. Promethazine [inn]
130. Promethazine [jan]
131. Promethazine [hsdb]
132. Schembl4700
133. Promethazine [vandf]
134. Lopac0_000899
135. Oprea1_758749
136. Bspbio_000676
137. Bspbio_002777
138. Kbiogr_001697
139. Kbioss_001348
140. Promethazine [mart.]
141. 4-27-00-01253 (beilstein Handbook Reference)
142. 73745-50-3
143. Divk1c_000005
144. Promethazine [who-dd]
145. Spbio_000799
146. Spbio_002895
147. (dimethylamino-2-propyl-10-phenothiazine Hydrochloride
148. Bpbio1_000744
149. Gtpl7282
150. Dtxsid7023518
151. Kbio1_000005
152. Kbio2_001348
153. Kbio2_003916
154. Kbio2_006484
155. Kbio3_001997
156. Pwwvaxiegoywee-uhfffaoysa-
157. Ex-a891
158. N,n-dimethyl-1-(10h-phenothiazin-10-yl)-2-propanamine
159. N,n-dimethyl-1-phenothiazin-10-yl-propan-2-amine
160. Ninds_000005
161. Hms2089e08
162. N,n-dimethyl-1-phenothiazin-10-ylpropan-2-amine Hydrochloride
163. Nsc30321
164. Tox21_110227
165. Bdbm50017696
166. Mfcd00066294
167. Akos015962127
168. Tox21_110227_1
169. 4182 R.p.
170. Ccg-109848
171. Db01069
172. Sdccgsbi-0050874.p005
173. Idi1_000005
174. Ncgc00015817-03
175. Ncgc00015817-04
176. Ncgc00015817-06
177. Ncgc00015817-08
178. Ncgc00015817-09
179. Ncgc00015817-10
180. Ncgc00015817-11
181. Ncgc00015817-12
182. Ncgc00015817-14
183. Ncgc00015817-17
184. Ncgc00015817-24
185. Ncgc00089735-02
186. Ncgc00089735-03
187. Ac-15939
188. Nci60_001878
189. Sbi-0050874.p004
190. Ab00053535
191. Ft-0700342
192. S5196
193. Wln: T C666 Bn Isj B1y1&n1&1
194. C07404
195. D00494
196. F17386
197. Ab00053535-12
198. Ab00053535_13
199. 10h-phenothiazine-10-ethanamine,n,n,b-trimethyl-
200. L000495
201. Q422970
202. J-690333
203. 10h-phenothiazine-10-ethanamine,n,.alpha.-trimethyl-
204. Sr-01000002993-10
205. 10-(2-dimethylamino-2-methylethyl)phenothiazine
206. (+/-)-10-(2-(dimethylamino)propyl)phenothiazine
207. N,n-dimethyl-1-(10h-phenothiazin-10-yl)-2-propanamine #
208. N,n,.alpha.-trimethyl-10h-phenothiazine-10-ethanamine
209. 10h-phenothiazine-10-ethanamine, N,n,.alpha.-trimethyl-, (+/-)-
| Molecular Weight | 284.4 g/mol |
|---|---|
| Molecular Formula | C17H20N2S |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 284.13471982 g/mol |
| Monoisotopic Mass | 284.13471982 g/mol |
| Topological Polar Surface Area | 31.8 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 298 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Promethegan |
| PubMed Health | Promethazine (Rectal) |
| Drug Classes | Antiemetic, Antivertigo, Gastrointestinal Agent |
| Drug Label | Each rectal suppository contains 12.5 mg or 25 mg promethazine HCl with ascorbyl palmitate, colloidal silicon dioxide, white wax, hard fat, and glyceryl monostearate. Promethazine HCl Suppositories are for rectal administration only.Promethazine HCl... |
| Active Ingredient | Promethazine hydrochloride |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | G And W Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Promethegan |
| PubMed Health | Promethazine (Rectal) |
| Drug Classes | Antiemetic, Antivertigo, Gastrointestinal Agent |
| Drug Label | Each rectal suppository contains 12.5 mg or 25 mg promethazine HCl with ascorbyl palmitate, colloidal silicon dioxide, white wax, hard fat, and glyceryl monostearate. Promethazine HCl Suppositories are for rectal administration only.Promethazine HCl... |
| Active Ingredient | Promethazine hydrochloride |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | G And W Labs |
Anti-Allergic Agents; Antiemetics; Antipruritics; Histamine H1 Antagonists; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
/Promethazine/ is indicated for the amelioration of allergic reactions to blood or plasma. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
/Promethazine/ is indicated in anaphylaxis as an adjunct to epinephrine and other standard measures after the acute symptoms have been controlled. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
/Promethazine/ is indicated for other uncomplicated allergic conditions of the immediate type when oral therapy is impossible or contraindicated. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
For more Therapeutic Uses (Complete) data for Promethazine (12 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: Respiratory Depression - Pediatrics. /Promethazine/ injection should not be used in pediatric patients less than 2 years of age because of the potential for fatal respiratory depression. Postmarketing cases of respiratory depression, including fatalities, have been reported with use of promethazine in pediatric patients less than 2 years of age. Caution should be exercised when administering promethazine injection to pediatric patients 2 years of age and older.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
/BOXED WARNING/ WARNING: Severe Tissue Injury, Including Gangrene. /Promethazine/ injection can cause severe chemical irritation and damage to tissues regardless of the route of administration. Irritation and damage can result from perivascular extravasation, unintentional intra-arterial injection, and intraneuronal or perineuronal infiltration. Adverse event reports include burning, pain, erythema, swelling, sensory loss, palsies, paralysis, severe spasm of distal vessels, thrombophlebitis, venous thrombosis, phlebitis, abscesses, tissue necrosis, and gangrene. In some cases, surgical intervention, including fasciotomy, skin graft, and/or amputation have been required. Due to the risks of intravenous injection, the preferred route of administration of promethazine injection is deep intramuscular injection. Subcutaneous injection is contraindicated.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1021
Promethazine injection is contraindicated in comatose states.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
For more Drug Warnings (Complete) data for Promethazine (55 total), please visit the HSDB record page.
3(?). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-222
Promethazine tablets and suppositories are indicated to treat rhinitis, allergic conjunctivitis, allergic reactions to blood or plasma, dermographism, anaphylactic reactions, sedation, nausea, vomiting, pain, motion sickness, and allergic skin reactions. Promethazine cough syrup with phenylephrine and codeine is indicated to relieve cough and upper respiratory symptoms, and nasal congestion associated with allergy or the common cold.
FDA Label
Promethazine is is a histamine H1 antagonist that can be used for it's ability to induce sedation, reduce pain, and treat allergic reactions. Promethazine's effects generally last 4-6h but can last up to 12h. Patients should be counselled regarding CNS and respiratory depression, reduce seizure threshold, and bone marrow depression.
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
D - Dermatologicals
D04 - Antipruritics, incl. antihistamines, anesthetics, etc.
D04A - Antipruritics, incl. antihistamines, anesthetics, etc.
D04AA - Antihistamines for topical use
D04AA10 - Promethazine
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AD - Phenothiazine derivatives
R06AD02 - Promethazine
Absorption
A 25mg dose of intramuscular promethazine reaches a Cmax of 22ng/mL. Intravenous promethazine reaches a Cmax of 10.0ng/mL, with a Tmax of 4-10h, and an AUC of 14,466ng\*h/mL. Oral promethazine is only 25% bioavailable due to first pass metabolism. Oral promethazine reaches a Cmax of 2.4-18.0ng/mL, with a Tmax of 1.5-3h, and an AUC of 11,511ng\*h/mL.
Route of Elimination
An intravenous dose of promethazine is 0.64% eliminated in the urine as the unchanged parent drug, 0.02-2.02% in the urine as desmethylpromethazine, 10% in the urine as promethazine sulfoxide.
Volume of Distribution
The volume of distribution of promethazine is approximately 970L or 30L/kg.
Clearance
The intravenous clearance of promethazine is approximately 1.14L/min. The renal clearance of promethazine is 5.9mL/min and the renal clearance of promethazine sulfoxide is 90.4mL/min.
Promethazine is well absorbed from the GI tract and from parenteral sites. Plasma concentrations of promethazine required for sedative effects are unknown. The onset of sedative effects occurs within 20 minutes following oral, rectal, or IM administration, and within 3-5 minutes following IV administration. The duration of sedative effects varies but may range from 2-8 hours depending on the dose and route of administration.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
Promethazine is widely distributed in body tissues. Compared with other organs, lower concentrations of the drug are found in the brain, but this concentration is higher than the plasma concentration.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
Promethazine has been reported to be 93% protein bound when determined by gas chromatography and as 76-80% bound when determined by high-performance liquid chromatography.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
Promethazine readily crosses the placenta. It is not known whether the drug is distributed into milk.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
For more Absorption, Distribution and Excretion (Complete) data for Promethazine (9 total), please visit the HSDB record page.
Promethazine is predominantly metabolized to promethazine sulfoxide, and minorly to desmethylpromethazine and a hydroxy metabolite. Hydroxylation of promethazine is predominantly mediated by CYP2D6.
Promethazine hydrochloride is metabolized in the liver, with the sulfoxides of promethazine and N-desmethylpromethazine being the predominant metabolites appearing in the urine.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
First order kinetics observed for oxidation of promethazine HCl in aqueous solution. Reaction rate was pH dependent up to pH 5. Cu ions increased rates as did Fe. Under anaerobic conditions, Cu and Fe were required for the reaction. Isolation of products carried out by tlc.
UNDERBERG WJ; J PHARM SCI 67(AUG) 1128-1138 (1978)
Incubation of promethazine (Ia) and desmethylpromethazine (Ib) with 9000g supernatant fractions of rabbit liver homogenate resulted in formation of N-dealkylated, N-oxygenated and ring-hydroxylated products. The N-oxidation products identified by t.l.c. and mass spectra using synthetic reference products are promethazine-N-oxide (IX) and the nitrone (VIII), which is believed to be formed chemically and metabolically from the metabolite N-hydroxydesmethylpromethazine (VII).
PMID:7314643 Clement B, Beckett AH; Xenobiotica 11 (9): 609-18 (1981)
To determine which cytochrome P450 form is involved in the promethazine [10-(2-dimethylaminopropyl) phenothiazine] metabolism, in vitro analysis using human liver microsomes were performed. Promethazine was mainly biotransformed to ring-hydroxylated, S-oxidized and N-demethylated metabolites. The promethazine hydroxylase in human liver microsomes was inhibited by SKF-525A, propranolol, sparteine, quinidine and anti-CYP2D6 serum suggesting involvement of a P450 related to CYP2D6. Lineweaver-Burk plots for the hydroxylation, S-oxidation and N-demethylation indicated that the hydroxylation occurred with a low K(m) value in human liver microsomes. Microsomes from genetically-engineered human B-lymphoblastoid cells expressing CYP2D6 hydroxylated promethazine most efficiently as compared to other P450 forms, indicating that it was the principal P450 responsible for the metabolism of promethazine in human liver microsomes. The inhibition of CYP2D6-catalysed bufuralol 1'-hydroxylase by various histamine H3 antagonists including promethazine suggested that promethazine and some other histamine H1 antagonists could be inhibitors of this P450 in human liver microsomes.
PMID:8946477 Nakamura K et al; Pharmacogenetics 6 (5): 449-57 (1996)
Promethazine has known human metabolites that include N-Methyl-1-(10H-phenothiazin-10-yl)propan-2-amine, Promethazine N-glucuronide, and Promethazine sulfoxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half life of promethazine is approximately 12-15h.
Following intravenous administration in healthy volunteers, the plasma half-life for promethazine has been reported to range from 9 to 16 hours. The mean plasma half-life for promethazine after intramuscular administration in healthy volunteers has been reported to be 9.8 +/- 3.4 hours.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
Half-life: 12 hours
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 21-6
Promethazine is a an antagonist of histamine H1, post-synaptic mesolimbic dopamine, alpha adrenergic, muscarinic, and NMDA receptors. The antihistamine action is used to treat allergic reactions. Antagonism of muscarinic and NMDA receptors contribute to its use as a sleep aid, as well as for anxiety and tension. Antagonism of histamine H1, muscarinic, and dopamine receptors in the medullary vomiting center make promethazine useful in the treatment of nausea and vomiting.
Promethazine is a phenothiazine derivative with potent sedative properties. Although the drug can produce either CNS stimulation or CNS depression, CNS depression manifested by sedation is more common with therapeutic doses of promethazine. The precise mechanism of the CNS effects of the drug is not known.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
Although it has been reported that the drug has slight antitussive activity, this may result from its anticholinergic and CNS depressant effects. In therapeutic doses, promethazine appears to have no substantial effect on the cardiovascular system. Although rapid IV administration of promethazine may produce a transient fall in blood pressure, blood pressure usually is maintained or slightly elevated when the drug is given slowly.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2608
Promethazine hydrochloride is a phenothiazine derivative which possesses antihistaminic, sedative, antimotion-sickness, antiemetic, and anticholinergic effects. Promethazine is a competitive H1 receptor antagonist, but does not block the release of histamine. Structural differences from the neuroleptic phenothiazines result in its relative lack (1/10 that of chlorpromazine) of dopamine antagonist properties.
US Natl Inst Health; DailyMed. Current Medication Information for PHENERGAN (promethazine hydrochloride) injection, solution (November 2009). Available from, as of June 25, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13417
The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2510
For more Mechanism of Action (Complete) data for Promethazine (18 total), please visit the HSDB record page.