
1. Mmc Lipid-based Prodrug (mlp)
2. Jnj-27548547
3. 3i8jkl1chb
4. Mitomycin C Lipid-based Prodrug (mlp)
5. 303983-00-8
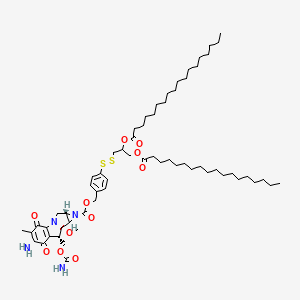
6. Azirino(2',3':3,4)pyrrolo(1,2-a)indole-1(2h)-carboxylic Acid, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1a,4,7,8,8a,8b-hexahydro-8a-methoxy-5-methyl-4,7-dioxo-, (4-((2,3-bis((1-oxooctadecyl)oxy)propyl)dithio)phenyl)methyl Ester, (1as,8s,8ar,8bs)-
7. Unii-3i8jkl1chb
8. Chembl4297496
9. Db15372
| Molecular Weight | 1139.6 g/mol |
|---|---|
| Molecular Formula | C62H98N4O11S2 |
| XLogP3 | 18.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 48 |
| Exact Mass | 1138.66735230 g/mol |
| Monoisotopic Mass | 1138.66735230 g/mol |
| Topological Polar Surface Area | 258 Ų |
| Heavy Atom Count | 79 |
| Formal Charge | 0 |
| Complexity | 1960 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |