

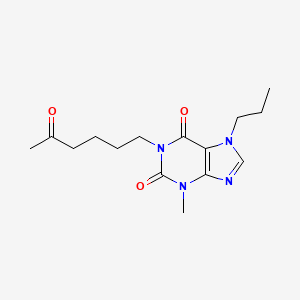

1. 1-(5'-oxohexyl)-3-methyl-7-propylxanthine
2. Hwa 285
3. Hwa-285
1. 55242-55-2
2. Propentophylline
3. Hextol
4. Hwa 285
5. Karsivan
6. Hwa-285
7. 3-methyl-1-(5-oxohexyl)-7-propylxanthine
8. 3-methyl-1-(5-oxohexyl)-7-propylpurine-2,6-dione
9. 3-methyl-1-(5-oxohexyl)-7-propyl-1h-purine-2,6(3h,7h)-dione
10. 3,7-dihydro-3-methyl-1-(5-oxohexyl)-7-propyl-1h-purine-2,6-dione
11. 1h-purine-2,6-dione, 3,7-dihydro-3-methyl-1-(5-oxohexyl)-7-propyl-
12. Nsc-752424
13. 5rta398u4h
14. 3-methyl-1-(5-oxohexyl)-7-propyl-3,7-dihydro-1h-purine-2,6-dione
15. Ncgc00015861-04
16. Albert-285
17. Dsstox_cid_25189
18. Dsstox_rid_80736
19. Dsstox_gsid_45189
20. Propentofylina
21. Propentofyllinum
22. Hoe-285
23. Propentofylina [inn-spanish]
24. Propentofyllinum [inn-latin]
25. Cas-55242-55-2
26. Sr-01000075642
27. Brn 1156290
28. Unii-5rta398u4h
29. Viviq
30. Propentofylline [inn:ban:jan]
31. 3arx
32. Poy
33. Propentofylline, Solid
34. 3as2
35. Spectrum3_001834
36. Lopac-p-9689
37. Propentofylline (jan/inn)
38. Propentofylline [mi]
39. Lopac0_001015
40. Schembl74602
41. Bspbio_003507
42. Propentofylline [inn]
43. Propentofylline [jan]
44. 5-26-14-00082 (beilstein Handbook Reference)
45. Mls001060796
46. Hwa285
47. Propentofylline [mart.]
48. Chembl1079905
49. Dtxsid4045189
50. Propentofylline [who-dd]
51. Chebi:32061
52. Kbio3_003012
53. Hms2197a12
54. Hms3263k11
55. Hms3355f02
56. Zinc1915505
57. Tox21 110247
58. Tox21_110247
59. Tox21_501015
60. Bdbm50492435
61. Nsc752424
62. Akos016003243
63. Tox21_110247_1
64. Ccg-205095
65. Db06479
66. Lp01015
67. Nsc 752424
68. Sdccgsbi-0050988.p003
69. Ncgc00015861-01
70. Ncgc00015861-02
71. Ncgc00015861-03
72. Ncgc00015861-06
73. Ncgc00015861-07
74. Ncgc00015861-08
75. Ncgc00015861-09
76. Ncgc00094307-01
77. Ncgc00094307-02
78. Ncgc00094307-03
79. Ncgc00178023-01
80. Ncgc00261700-01
81. Smr000486263
82. Sbi-0050988.p002
83. 1-(5-oxohexyl)-3-methyl-7-propylxanthine
84. Db-052700
85. Hy-107203
86. Cs-0027629
87. Eu-0101015
88. Ft-0630675
89. D01630
90. P 9689
91. 242p552
92. Q2888695
93. Sr-01000075642-1
94. Sr-01000075642-5
95. Brd-k59273480-001-01-5
| Molecular Weight | 306.36 g/mol |
|---|---|
| Molecular Formula | C15H22N4O3 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 306.16919058 g/mol |
| Monoisotopic Mass | 306.16919058 g/mol |
| Topological Polar Surface Area | 75.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 454 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in alzheimer's disease.
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BC - Xanthine derivatives
N06BC02 - Propentofylline
Propentofylline is a xanthine derivative and phosphodiesterase inhibitor with purported neuroprotective effects. It inhibits both phosphodiesterase and adenosine uptake. Phosphodiesterase has shown to associated with age-related memory impairment and Alzheimer's disease. -Amyloid protein 142 (42) can induce apoptosis in the cultured hippocampal neurons, suggesting that it plays an important role in causing neurodegeneration in Alzheimer's disease. Propentofylline is also capable of activating a cAMPPKA system, depressing the caspase cascade and modifying Bcl-2 family proteins. Propentofylline blocked both the apoptotic features induced by 42 and further induced an anti-apoptotic protein, Bcl-2. It suggests that the protection of propentofylline on the 42-induced neurotoxicity is caused by enhancing anti-apoptotic action through cAMPPKA system.
