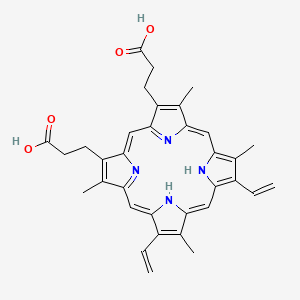

1. Ppix
2. Protoporphyrin
3. Protoporphyrin Ix Dihydride
4. Protoporphyrin Ix, Disodium Salt
1. Protoporphyrin
2. 553-12-8
3. Ooporphyrin
4. Protoporpyrin Ix
5. Porphyrinogen Ix
6. Protoporphyrin Ix Disodium
7. Kammerer's Prophyrin
8. Ppix
9. H2ppix
10. Kammerer's Porphyrin
11. Mls001074731
12. 3-[18-(2-carboxyethyl)-8,13-bis(ethenyl)-3,7,12,17-tetramethyl-22,23-dihydroporphyrin-2-yl]propanoic Acid
13. Chebi:15430
14. Nsc2632
15. Nsc-2632
16. 3,7,12,17-tetramethyl-8,13-divinyl-2,18-porphinedipropionic Acid
17. Smr000127405
18. 21h,23h-porphine-2,18-dipropanoic Acid, 7,12-diethenyl-3,8,13,17-tetramethyl-
19. Chembl267548
20. 3,3'-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)di(propionic Acid)
21. Protoporphyrin Ix Containing Fe
22. C2k325s808
23. 2,18-porphinedipropionic Acid, 3,8,13,17-tetramethyl-7,12-divinyl-
24. 21h,23h-porphine-2,18-dipropanoic Acid,7,12-diethenyl-3,8,13,17-tetramethyl-
25. 3,3'-(3,7,12,17-tetramethyl-8,13-divinyl-21h,23h-porphine-2,18-diyl)-bis-propionic Acid
26. Protoporphyrinix
27. 3,7,12,17-tetramethyl-8,13-divinylporphyrin-2,18-dipropanoic Acid
28. 7,12-diethenyl-3,8,13,17-tetramethylporphyrin-2,18-dipropanoic Acid
29. 3,3'-(3,8,13,17-tetramethyl-7,12-divinylporphyrin-2,18-diyl)dipropionic Acid
30. Sr-01000076084
31. Nsc 2632
32. Einecs 209-033-7
33. Protoporphyrin-9
34. Unii-c2k325s808
35. Heme-b
36. Protoporphyrin-"ix"
37. Spectrum_001300
38. Hem
39. Spectrum2_001016
40. Spectrum3_001440
41. Spectrum4_000419
42. Spectrum5_001303
43. Cid_4971
44. Lopac0_000973
45. Schembl25719
46. Bspbio_003180
47. Kbiogr_000718
48. Kbioss_001780
49. Protoporphyrin Ix, >=95%
50. Divk1c_000074
51. Schembl805850
52. Spectrum1501111
53. Spbio_001171
54. Protoporphyrin Ix [mi]
55. Schembl3287054
56. Protoporphyrin [who-dd]
57. Chembl1325592
58. Chembl1618319
59. Chembl1907972
60. Chembl1907974
61. Chembl4463327
62. Dtxsid4048353
63. Schembl14552654
64. Bdbm51757
65. Hms500d16
66. Kbio1_000074
67. Kbio2_001780
68. Kbio2_004348
69. Kbio2_006916
70. Kbio3_002400
71. Ninds_000074
72. Hms1921f05
73. Hms2271b12
74. Hms3263c07
75. Pharmakon1600-01501111
76. Hy-b1247
77. Tox21_500973
78. Bdbm50523755
79. Ccg-39673
80. Mfcd00151109
81. Nsc757839
82. Cs-4849
83. Db02285
84. Lp00973
85. Nsc-757839
86. Sdccgmls-0066712.p001
87. Sdccgsbi-0050946.p004
88. Idi1_000074
89. Ncgc00015844-01
90. Ncgc00015844-02
91. Ncgc00015844-03
92. Ncgc00015844-04
93. Ncgc00015844-05
94. Ncgc00015844-07
95. Ncgc00015844-08
96. Ncgc00015844-12
97. Ncgc00094273-01
98. Ncgc00094273-02
99. Ncgc00094273-03
100. Ncgc00162303-01
101. Ncgc00185010-01
102. Ncgc00261658-01
103. As-17461
104. Sbi-0050946.p003
105. Eu-0100973
106. Ft-0696783
107. Protoporphyrin Ix [mesh: Protoporphyrin Ix]
108. J1.756.122g
109. 2, 3,8,13,17-tetramethyl-7,12-divinyl-
110. C02191
111. C75594
112. P 8293
113. Ab00052204_02
114. 553p128
115. A870226
116. Q619815
117. Sr-01000763747
118. Sr-01000076084-1
119. Sr-01000076084-4
120. Sr-01000076084-5
121. Sr-01000763747-3
122. Brd-k26813314-001-04-4
123. 1,3,5,8-tetramethyl-2,4-divinylporphine-6,7-dipropionate
124. 21h,18-dipropanoic Acid, 7,12-diethenyl-3,8,13,17-tetramethyl-
125. 3,3'-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)di
126. 2,18-porphinedipropionic Acid, 3,8,13,17-tetramethyl-7,12-divinyl- (8ci)
127. 3,3'-(3,7,12,17-tetramethyl-8,13-divinyl-21h,23h-porphine-2,18-diyl)-bis-propionate
128. 3,3'-(7,12-diethenyl-3,8,13,17-tetramethylporphyrin-2,18-diyl)dipropanoic Acid
129. 8,13-divinyl-3,7,12,17-tetramethyl-21h,23h-porphyrin-2,18-bispropanoic Acid
130. 3-[(1z,4z,9z,15z)-18-(2-carboxy-ethyl)-3,7,12,17-tetramethyl-8,13-divinyl-porphyrin-2-yl]-propionic Acid
131. 3-[(1z,4z,9z,15z)-18-(2-carboxyethyl)-3,7,12,17-tetramethyl-8,13-divinyl-21,23-dihydroporphyrin-2-yl]propanoic Acid
132. 3-[18-(2-carboxyethyl)-3,7,12,17-tetramethyl-8,13-divinyl-22,23-dihydroporphin-2-yl]propionic Acid
133. 3-[8,13-bis(ethenyl)-18-(3-hydroxy-3-oxopropyl)-3,7,12,17-tetramethyl-22,23-dihydroporphyrin-2-yl]propanoic Acid
| Molecular Weight | 562.7 g/mol |
|---|---|
| Molecular Formula | C34H34N4O4 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 562.25800558 g/mol |
| Monoisotopic Mass | 562.25800558 g/mol |
| Topological Polar Surface Area | 132 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)