

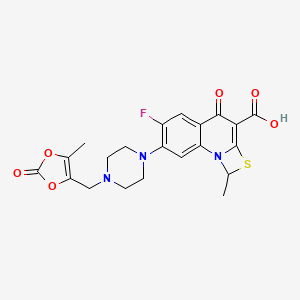

1. 6-fluoro-1-methyl-7-(4-(5-methyl-2-oxo-1,3-dioxelen-4-yl)methyl-1-piperazinyl)-4-oxo-4h-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic Acid
2. Nm 441
3. Nm-441
4. Nm441
1. 123447-62-1
2. Pruvel
3. Nm441
4. Quisnon
5. Nm-441
6. Prulifloxacin [inn]
7. Sword
8. Nm 441
9. Prulifloxacin (hydrochloride)
10. 6-fluoro-1-methyl-7-(4-((5-methyl-2-oxo-1,3-dioxol-4-yl)methyl)piperazin-1-yl)-4-oxo-1,4-dihydro-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic Acid
11. J42298iesw
12. Nsc-759833
13. 6-fluoro-1-methyl-7-[4-[(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl]piperazin-1-yl]-4-oxo-1h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic Acid
14. Ncgc00164615-01
15. Prulifloxacin 100 Microg/ml In Acetonitrile
16. Dsstox_cid_26480
17. Dsstox_rid_81651
18. Dsstox_gsid_46480
19. 1h,4h-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic Acid, 6-fluoro-1-methyl-7-(4-((5-methyl-2-oxo-1,3-dioxol-4-yl)methyl)-1-piperazinyl)-4-oxo-
20. 1h,4h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic Acid, 6-fluoro-1-methyl-7-[4-[(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl]-1-piperazinyl]-4-oxo-
21. 6-fluoro-1-methyl-7-(4-((5-methyl-2-oxo-1,3-dioxol-4-yl)methyl)piperazin-1-yl)-4-oxo-1h,4h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic Acid
22. Ccris 7686
23. Cas-123447-62-1
24. Unii-j42298iesw
25. Pufloxacin Dioxolil
26. 6-fluoro-1-methyl-7-{4-[(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl]piperazin-1-yl}-4-oxo-4h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic Acid
27. Prulifloxacin [mi]
28. Prulifloxacin [jan]
29. Mls006011767
30. Prulifloxacin [mart.]
31. Prulifloxacin [who-dd]
32. Chembl422648
33. Nad-441a
34. Opt-99
35. Schembl1650794
36. Dtxsid0046480
37. Chebi:32071
38. Bcp12727
39. Hy-b0024
40. Tox21_112235
41. Bbl034010
42. Mfcd00864847
43. S2071
44. Stk626148
45. Akos005559236
46. Nm441;af 3013
47. Prulifloxacin (nm441, Af 3013)
48. Tox21_112235_1
49. Ac-2012
50. Af 3012
51. Db11892
52. Nsc 759833
53. Sb18983
54. Ncgc00164615-02
55. Ncgc00164615-03
56. (+-)-7-(4-((z)-2,3-dihydroxy-2-butenyl)-1-piperazinyl)-6-fluoro-1-methyl-4-oxo-1h,4h-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic Acid, Cyclic Carbonate
57. As-13607
58. Smr000046722
59. Ft-0651733
60. P2058
61. F20576
62. 447p621
63. Prulifloxacin, >=98% (perchloric Acid Titration)
64. Sr-01000872596
65. Prulifloxacin, Antibiotic For Culture Media Use Only
66. Q-101929
67. Q3924793
68. Sr-01000872596-1
69. Brd-a92341659-001-01-6
70. 6-fluoro-1-methyl-7-[4-[(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl]piperazin-4-ium-1-yl]-4-oxo-1h-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate
| Molecular Weight | 461.5 g/mol |
|---|---|
| Molecular Formula | C21H20FN3O6S |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 461.10568470 g/mol |
| Monoisotopic Mass | 461.10568470 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 931 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA17 - Prulifloxacin